Published By VITA 1600 Wilson Boulevard, Suite 500 Arlington, Virginia 22209 USA Tel: 703/276-1800 . Fax: 703/243-1865 Internet: pr-info@vita.org
Understanding Ethanol Fuel Production and Use ISBN: 0-86619-203-4 [C]1984, Volunteers in Technical Assistance
PREFACE
This paper is one of a series published by Volunteers in Technical Assistance to provide an introduction to specific state-of-the-art technologies of interest to people in developing countries. The papers are intended to be used as guidelines to help people choose technologies that are suitable to their situations. They are not intended to provide construction or implementation details. People are urged to contact VITA or a similar organization for further information and technical assistance if they find that a particular technology seems to meet their needs.
The papers in the series were written, reviewed, and illustrated almost entirely by VITA Volunteer technical experts on a purely voluntary basis. Some 500 volunteers were involved in the production of the first 100 titles issued, contributing approximately 5,000 hours of their time. VITA staff included Leslie Gottschalk as primary editor, Julie Berman handling typesetting and layout, and Margaret Crouch as project manager.
Cliff Bradley and Ken Runnion, co-authors of this paper, specialize in alcohol fuel production technologies. Bradley is a microbiologist and Runnion a chemical engineer at Renewable Technologies, Inc. They have published several practical manuals and booklets in the field, and are currently researching and developing new methods of improving the starch hydrolysis process required for alcohol fuel production. Reviewers Kenneth Brunot, C. Gene Haugh, and Daniel Ingold are also specialists in the area. Brunot, senior vice president for Wright Technology, was formerly president of Wright Energy Nevada Corporation, where he specialized in studies relating to ethanol production using geothermal energy for process energy requirements. Haugh heads the Department of Agricultural Engineering at Virginia Polytechnic Institute. Ingold is a biophysicist by training and a research engineer at Appropriate Technology Corporation.
VITA is a private, nonprofit organization that supports people working on technical problems in developing countries. VITA offers information and assistance aimed at helping individuals and groups to select and implement technologies appropriate to their situations. VITA maintains an international Inquiry Service, a specialized documentation center, and a computerized roster of volunteer technical consultants; manages long-term field projects; and publishes a variety of technical manuals and papers.
I. INTRODUCTION
This paper describes the production and use of ethanol (ethyl alcohol) as a liquid fuel. The production of ethanol is a well-established technology; however, the use of ethanol as a liquid fuel is a complex subject.
Ethanol was one of the first fuels used in automobile engines. It was used extensively in Germany during World War II and also in Brazil, the Philippines, and the United States. During the postwar period, as petroleum supplies became cheap and abundant, gasoline largely replaced ethanol as an automotive fuel. Not until the 1970s, when the supply of oil was restricted, did ethanol re-emerge as an alternative to or extender for petroleum-based liquid fuels (ethanol as an extender is added to these fuels to increase their volume). Today, 12 countries produce and use a significant amount of ethanol. In Brazil, for example, one third of that country's automobiles uses pure ethanol as fuel; the remaining two thirds use mixtures of gasoline and ethanol. France, the United States, Indonesia, the Philippines, Guatemala, Costa Pica, Argentina, the Republic of South Africa, Kenya, Thailand, and Sudan are other countries with government or private ethanol fuel programs. The programs are designed to reduce a country's dependence on costly imported fuel and to assist in creating a new domestic fuel industry.
Pure ethanol can replace gasoline in modified spark-ignition engines, or it can be blended with gasoline at up to 20 percent concentration to fuel unmodified gasoline engines. Blending serves two purposes: (1) it extends gasoline supplies, and (2) as an octane enhancer, it replaces lead compounds in gasoline. Ethanol can also be used in modified diesel (compression ignition) engines; however, this is not common.
The production and use of fuel ethanol can indirectly serve a variety of needs. On a national level, ethanol can improve balance of payments by displacing imported petroleum with domestically produced fuel. This may also provide increased rural employment and alternative markets for agricultural commodities. On a community or individual level, ethanol fuel production is often viewed as a means to become independent of purchased fuels, to keep money within the local economy, and to provide an assured fuel supply in the event of shortages of petroleum fuels.
II. OPERATING PRINCIPLES
ETHANOL PRODUCTION
Ethanol fuel production is a combination of biological and physical processes. Ethanol is produced by fermentation of sugars with yeast. It is concentrated to fuel grade by distillation. Figure 1
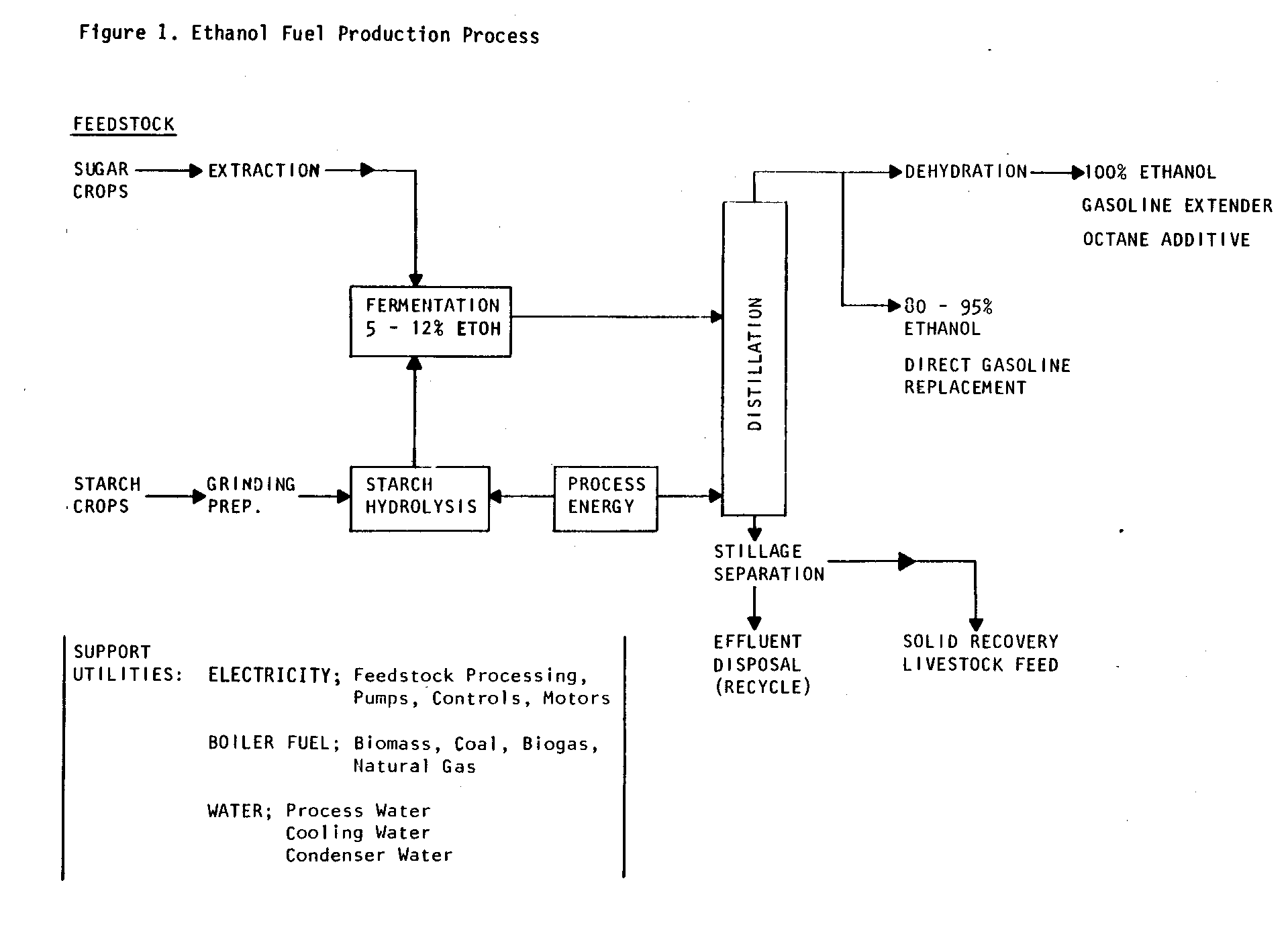
is a schematic representation of the principal steps in fuel ethanol production.
Feedstocks, the base raw materials, are either sugar or starch-containing crops. These "Biomass Fuel Crops" (tubers and grains) commonly include sugar beets, potatoes, corn, wheat, barley, Jerusalem artichokes, and sweet sorghum. Sugar crops such as sugar cane, sugar beets, or sweet sorghum are extracted to produce a sugar-containing solution that can be directly fermented by yeast. Starch feedstocks, however, must be carried through an additional conversion step.
Starch is a long "chain" polymer of glucose (i.e., many glucose polymer units bonded in a chain). Starches cannot be directly fermented to ethanol. They must first be broken down into the simpler glucose units through a process of hydrolysis. In the hydrolysis step, starch feedstocks are ground and mixed with water to produce a mash typically containing 15 to 20 percent starch. The mash is then cooked at boiling point or above and treated in sequence with two enzyme preparations. The first enzyme hydrolyzes starch molecules to short chains; the second enzyme hydrolyzes the short chains to glucose. The mash is then cooled to 30[degrees] C, and yeast is added.
Yeasts are microorganisms that produce ethanol. These microorganisms are capable of converting sugar into alcohol by a biological process called fermentation. The following equation shows the
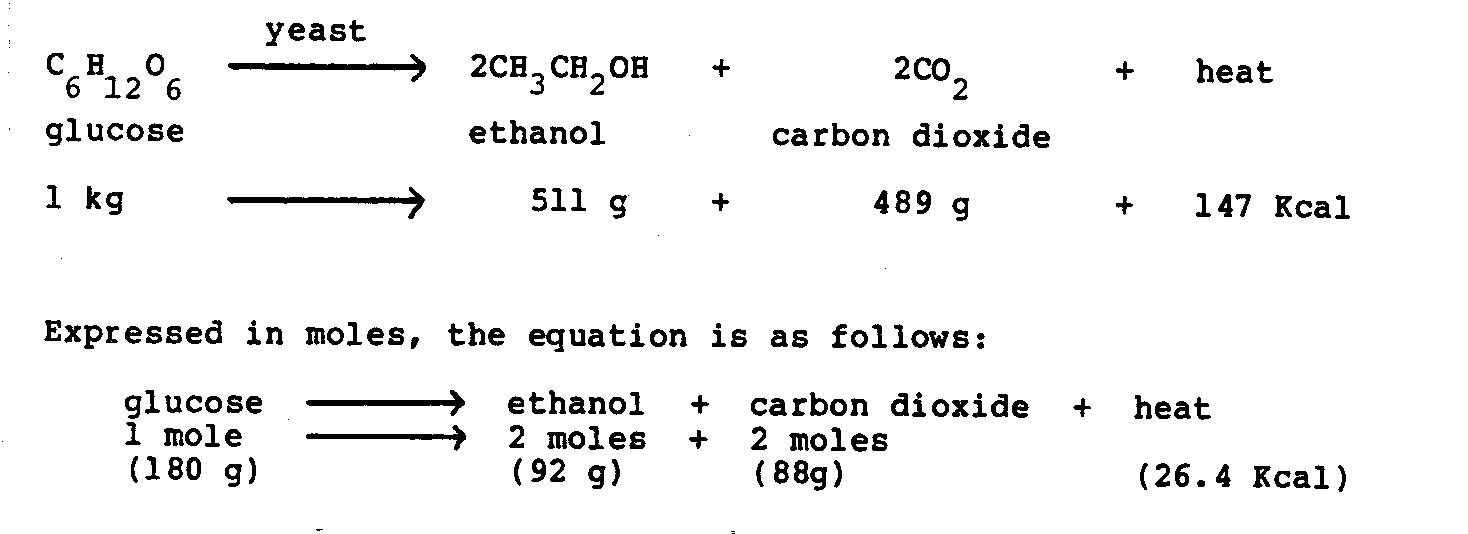
basic biological reaction in the conversion by fermentation of one kilogram of glucose to ethanol, carbon dioxide, and heat:
Theoretically, the maximum conversion efficiency of glucose to ethanol is 51 percent on a weight basis. However, some glucose is used by the yeast for the production of cell mass and for metabolic products other than ethanol. In practice, between 40 and 48 percent of glucose is converted to ethanol. With a 45-percent fermentation efficiency, 1,000 kilograms of fermentable sugar produce about 570 liters of pure ethanol. Conversely, about 1,800 kilograms of fermentable sugar are required to produce 1,000 liters of ethanol. Mash typically contains between 50 and 100 grams of ethanol per liter (5 to 10 percent weight per volume) when fermentation is complete.
Ethanol is separated from mash by distillation--a physical process in which the components of a solution (in this case, water and ethanol) are separated by differences in boiling point or vapor pressure.
Ethanol and water form an azeotrope, or constant boiling solution, of about 95 percent alcohol and five percent water. The five percent water cannot be separated by conventional distillation. The production of pure, water-free (anhydrous) ethanol requires a dehydration step following distillation. Dehydration, a relatively complex step in ethanol fuel production, is accomplished in one of two ways. The first method uses a third liquid, most commonly benzene, which is added to the ethanol/ water mixture. This changes the boiling characteristics of the solution, allowing separation of anhydrous ethanol. The second method employs molecular sieves that selectively absorb water on the basis of the difference in molecular size between water and ethanol.
The non-fermentable solids in distilled mash (stillage) contain variable amounts of fiber and protein, depending on the feedstock. The liquid may also contain soluble protein and other nutrients. The recovery of the protein and other nutrients in stillage for use as livestock feed can be essential for economical ethanol fuel production. Protein content will vary with feedstock. Some grains (e.g., corn, barley) yield a solid by-product --distillers dried grains (DDG)--that ranges from 25 to 30 percent protein and makes an excellent feed for livestock. If the processing equipment is constructed of stainless steel and processing is carried out under well-controlled conditions, the protein by-products can also be consumed by humans.
The production of ethanol also produces liquid effluent, which is a potential pollution problem. About 9 liters of effluent are produced for each liter of ethanol. In well-designed plants, some of the effluent may be recycled. Effluent can have a high Biological Oxygen Demand (BOD), which is a measure of organic water pollution potential, and it is acidic. It requires treatment before discharge. Treatment requirements depend on feedstock and local pollution control regulations. Because of the acid content, care must be taken if the effluent is spread over fields.
ETHANOL END USE
Ethanol is a high-quality, stable liquid. Some of the chemical and physical properties of ethanol are summarized in Table 1.
Table 1. Summary of Ethanol Properties
Property Ethanol
Chemical Formula.............................. [C.sub.2][H.sub.5]OH
Molecular Weight.............................. 46.07
Density (20[degrees] C)....................... 0.791 g/cc
Boiling Point [a]............................. 78.5[degrees] C
Heat of Combustion [b]........................ 5625 Kcal/1
Heat of Vaporization [c]...................... 9.225 Kcal/mole
Octane Rating................................. 106-108
Stoichiometric Air/Fuel Ratio [d]............. 9/1
[a] Boiling point is the temperature at which a liquid changes phase and becomes a gas; the point at which the vapor pressure of the liquid equals the vapor pressure of the system.
[b] Heat of combustion is the amount of heat given off when a unit quantity of any hydrocarbon (e.g., ethanol) is burned to carbon dioxide and water.
[c] Heat of vaporization is the heat input required to change liquid at its boiling point to a vapor at the same temperature (e.g., water at 100[degrees] C to steam at 100[degrees] C).
[d] The stoichiometric air/fuel ratio is the amount of air necessary completely to oxidize (burn) the fuel.
Ethanol Use in Engines
Ethanol is most commonly used in transportation and agriculture to fuel internal combustion, four-cycle, spark-ignition engines. It is used as a direct replacement for gasoline, or it is blended with gasoline as an extender and octane booster.
The use of ethanol to replace gasoline requires modifications to the carburetor, fuel system components, and often the compression ratio. The efficient conversion of existing gasoline engines requires skilled, knowledgeable technicians.
Engines specifically designed and manufactured to operate on ethanol fuel will generally be more efficient than modified gasoline engines. Ethanol concentrations of between 80 and 95 percent can be used as fuel, which eliminates the need for sophisticated dehydration systems and simplifies distillation. In many cases, the conversion of engines to operate on ethanol may be simpler and more cost efficient than ethanol dehydration. The disadvantage of engine conversion is that vehicle travel distance is limited by the available supply and distribution of ethanol.
Some "dual fuel" systems--that is, engines with a carburetor that can operate either on ethanol or on gasoline--have been developed on a limited basis. In Brazil, a significant portion of the transportation fleet uses ethanol fuel in automobiles with specially designed engines, manufactured by major international automobile companies.
In unmodified engines, ethanol can replace up to 20 percent of the gasoline. Blending ethanol with gasoline extends the gasoline supply, and improves the quality of gasoline by increasing its octane value. As an octane enhancer, ethanol can replace lead compounds in gasoline. There are advantages to using gasoline/ ethanol blends rather than pure ethanol. Blends do not require engine modification. In this way, ethanol can be integrated rapidly with existing gasoline supply and distribution systems. Replacing lead compounds with ethanol removes one of the principal air pollution problems associated with gasoline.
The disadvantage of using ethanol/gasoline blends is that the ethanol must be anhydrous, requiring a dehydration step in production. If non-anhydrous ethanol is mixed with gasoline, the blends will separate into a gasoline phase and a water/ethanol phase, causing erratic engine performance.
In addition to its use in gasoline-fueled automobiles and in truck or tractor engines, ethanol can be used in other types of engines. For example, small, four-cycle gasoline engines found in small-scale agricultural equipment (e.g., tillers, small tractors) can often burn 80 to 95 percent ethanol as a direct replacement for gasoline. Such engines fed by ethanol require minimal modifications.
The use of ethanol in specially designed two-cycle engines has been demonstrated on a limited basis. The problem of using ethanol in these engines is that the ethanol does not blend well with lubricating oil. To get around this problem, research is under way to find lubricating oils that are not affected by ethanol.
Though ethanol use in diesel-fueled engines is feasible, it has its limitations. Ethanol does not ignite under compression and does not mix well with diesel fuel. Therefore, ethanol cannot be used as a direct replacement for diesel fuel or blended with diesel fuel for use in compression ignition engines. Ethanol can be used as a replacement for diesel fuel only if the engine is fitted with glow plugs.
Ethanol can be used in supercharged diesel engines for up to about 25 percent of the total fuel. This is done by carrying the ethanol in a separate fuel tank and injecting it into the diesel engine through a supercharger airstream.
Ethanol can also replace aviation fuel in aircraft engines.
Ethanol Use in Appliances
Ethanol can be used in a variety of cooking, heating, and lighting appliances. In some cases, ethanol can be used in modified appliances designed for conventional fuels. In other cases, appliances designed specifically for ethanol fuel are required.
III. PLANT DESIGN VARIATIONS
This section describes briefly the processes and equipment necessary for each principal step in ethanol fuel production. It also provides a general discussion of the economics of ethanol fuel production. It is not meant to provide specific information on plant design.
Processes and equipment vary greatly, depending on feedstock, the need for starch hydrolysis, ethanol end use, available support utilities, process energy source, by-product use, and plant scale.
FEEDSTOCK PROCESSING
Plant design studies indicate that an economy of scale exists for a 30,000,000 gal/year plant producing hydrated (190 proof) ethanol and co-generating, i.e., utilizing on-site gas turbine generator sets fueled with hydrated ethanol to provide associated power needs for the plant. The turbine exhaust gas could be used to obtain high-pressure steam and the spent hot turbine exhaust gas could be used in process by-product drying operations. Provision to produce process by-products (distillers dried grains (DDG), carbon dioxide, and fusel oil components should be included in the overall design in order to maximize cost effectiveness.
The type of feedstock chosen for ethanol fuel production has a significant impact on plant design. Ethanol is produced from a variety of sugar- or starch-containing crops, with modifications in the design of the feedstock preparation processes. The modifications are required to accommodate the physical properties of the feedstock, as well as the nature of the carbohydrate (i.e., sugar versus starch).
Preparation equipment is necessary to grind, pulverizer or extract the feedstock before it can be processed. Milling equipment for feedstock preparation varies, depending on such characteristics of the feedstock as moisture content, physical structure, and fiber content.
Starch Hydrolysis
Starch-containing feedstocks require starch hydrolysis equipment including tanks, heating and cooling systems, agitation systems, transfer pumps, and monitoring instruments. Starchy feedstocks have to be ground before hydrolysis to a particle size that can pass through a 20-mesh screen.
Steam circulated through heat exchangers is the most common means of heating the mash; therefore, starch hydrolysis heating requirements must be included in plant boiler capacity.
Cooling the mash from boiling to fermentation temperature (about 30[degrees] C) generally the determining factor in heat exchanger design. This is especially true in tropical climates where the ambient temperature of the cooling water is relatively high.
The agitation systems for starch hydrolysis tanks must be adequate to mix viscous (thick) starch solutions efficiently. When starch is heated in water, it forms a very thick gel. Starch gelatinization is essential for efficient enzymatic hydrolysis. Thorough mixing of gelled starch mash is necessary to ensure efficient heat exchange and enzyme activity.
Monitoring equipment for starch hydrolysis includes thermometers to measure mash temperature and steam temperature, and pressure gauges to measure mash pressure if pressurized starch hydrolysis systems are used. Tests to measure the efficiency of starch hydrolysis are also necessary. Generally speaking, the feedstock is the most important element in determining the economics of ethanol production, and inefficient starch hydrolysis can have a major economic impact on ethanol production.
Starch hydrolysis systems are of two general types: batch systems and continuous systems. Batch systems consist of tanks that are sized in relation to fermentation tank capacity and holding time. The tank is equipped with heat exchangers, usually internal coils, that circulate steam and cooling water. The mash is agitated by a motor equipped with gear reduction and mixing impellers. Transfer pumps capable of handling a high level of solids are used to transfer the mash of fermentation tanks. With very viscous feedstocks, heat exchange and mash agitation are accomplished by pumping the mash through an external heat exchanger and back into the tank. Batch systems are operated by filling the tank, carrying out the multistep process of enzyme hydrolysis, and then pumping the entire mash volume into fermenters.
Continuous starch hydrolysis systems require more sophisticated equipment, but they are usually more efficient. Continuous systems generally use "jet cookers," in which mash and stem are mixed under pressure at temperatures of 105 to 150[degrees] C. Water, feedstock, and enzyme are fed into a premix tank at a controlled rate, heated, and pumped under pressure through the jet. The mash is kept at high pressure and high temperatures for a few minutes, then released from the cooker to holding tanks, where it is cooled and additional enzyme is added. The mash is then transferred to fermenters. The high pressure and temperature of these systems result in more efficient starch gelatinization and hydrolysis. These systems require high-pressure boilers and relatively sophisticated systems for maintaining the mash under pressure. Equipment is sized on the basis of plant fermenter capacity and mash residence time in cookers.
Fermentation
Fermentation takes place in tanks equipped with agitation and heat exchangers to remove the heat generated by fermentation. Tank size is based on the concentration of sugar in the mash, fermentation time, final ethanol concentration, and plant production rate.
Final mash ethanol concentration is a direct function of mash sugar concentration. Within the limits of feedstocks and yeast ethanol tolerance, higher ethanol concentrations are desirable. Maximum mash ethanol concentration is about 10 percent weight per volume. At concentrations higher than 10 percent, yeast are killed. Generally, feedstocks with high moisture content and sugar or starch concentrations less than 20 percent can be fermented without dilution. Feedstocks with high starch or sugar concentrations require dilution. The sugar will be wasted if the concentration is in excess of the amount necessary to produce the maximum amount of ethanol tolerated by the yeast.
Fermentation typically requires from 12 to 72 hours depending on the amount of yeast used to start fermentation and mash sugar concentration. Plants are usually equipped with multiple fermentation tanks run on staggered schedules to provide a continuous supply of fermented mash for distillation.
One of the most significant problems in ethanol production, especially in small-scale plants, is contamination of mash by bacteria. Bacteria utilize sugars that would otherwise be converted to ethanol. Good plant design and efficient fermentation can control contamination wihout resorting to costly sterilization systems.
Distillation
Distillation systems can either be batch or continuous. Choosing one system or the other is based on plant scale. Both types require heating systems, usually steam (which can be from low-pressure boilers), a distillation column, and a condenser. Figure 2 shows schematics of these two types of systems.
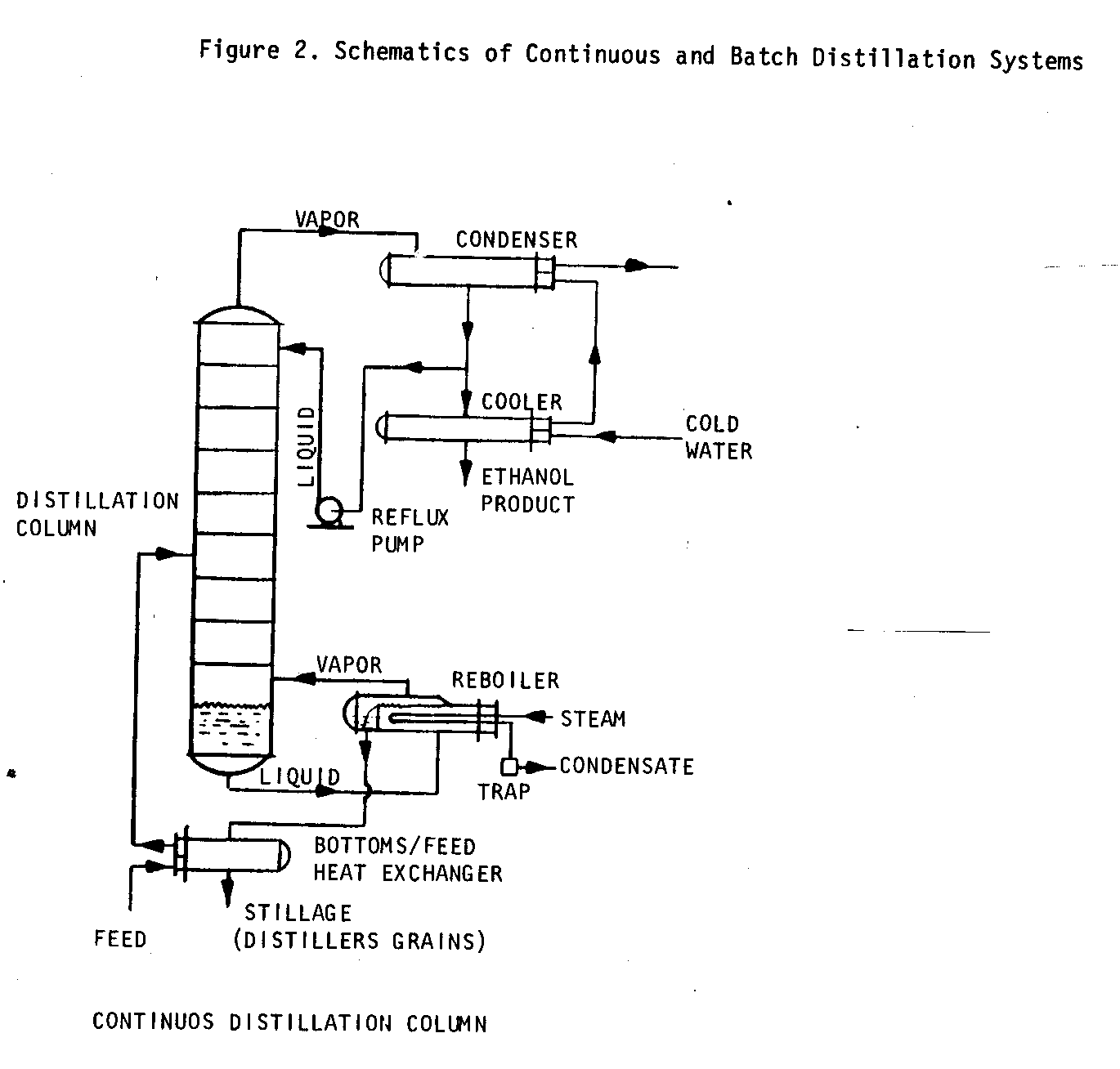
Distillation column size and ethanol production rate are based on the concentration of ethanol in the fermented mash, fermentation capacity, and production schedules. Small-scale plants--up to about 100,000 liters annual ethanol production--can efficiently use batch distillation systems. In batch systems, the entire mash volume is passed, or charged, to a large vessel called a still, which is then heated. The vapors are allowed to pass into the distillation column. Though batch systems are less efficient than continuous feed distillation systems, they are much easier to build and operate.
In continuous feed systems, fermented mash is pumped at a controlled rate into the distillation column, with heat introduced at the bottom of the column. Provision is made at the top of the column to feed unprocessed mash back through the system. Continuous feed columns should be used in large-scale plants where the improved efficiency justifies the added complexity.
Dehydration
The intended use of the ethanol determines the need for dehydration systems to remove the five percent water that cannot be separated by distillation. If ethanol is to be blended with gasoline, dehydration is required. The presence of water in ethanol gasoline-blends results in phase separation in storage or fuel tanks. Dehydration is not required if ethanol is to be used to replace gasoline. Ethanol can be used directly in modified engines at concentrations of between 80 and 95 percent.
By-product Recovery
Solid by-products are recovered from stillage with solid/liquid separation equipment. This equipment can range from simple screens to such complex equipment as centrifuges or vacuum filters. Soluble protein in thin stillage can be recovered by evaporation. If by-products are to be stored or transported significant distances, drying is necessary. Stillage with high moisture content can often be fed directly to livestock at or near the site of ethanol production with minimal separation or processing.
Effluent Treatment
Production of each volume of fuel ethanol will generate about nine volumes of effluent. A portion of the effluent can be recycled and used to dilute high concentration feedstocks. However, even if the effluent is recycled, it can still cause a significant pollution problem. To avoid pollution of surface water or ground water, the effluent must undergo microbiological degradation; that is, the harmful organic matter contained in the effluent must be broken down before the effluent is disposed of. This is done anaerobically, aerobically, or by a sequential combination of the two methods. Effluent degradation is usually done in a simple treatment pond, followed by a stabilization pond, if necessary. Alternatively, the effluent can be fed to biogas digesters, combining energy production with waste treatment.
Utilities
Ethanol production requires water, boiler fuel, and transportation for feedstock, ethanol, and by-products. Electricity may be used to run pumps, stirring motors, process controls, and instrumentation, but there are many units throughout the world that produce up to 10,000 gallons per year without electricity. Water is required for mash dilution and cooling in heat exchangers used with starch hydrolysis systems, fermenters, and condensers.
Boilers used to generate process steam require a low-cost, low-quality fuel such as natural gas, biogas, biomass, coal, residual oil, or bagasse (bagasse is crushed sugar cane or beet refuse from sugar making). High-quality liquid fuels or electricity are uneconomical and inefficient for use as boiler fuel. Finally, the feedstock needs to be transported to the plant; and the products, both ethanol and by-product animal feed, need to be transported to the point of use.
The process energy requirements for ethanol fuel production vary widely depending on equipment, process design, and feedstock. The production of one liter of ethanol with a heating value of 5,625 Kcal/liter would typically require 800 to 1,200 Kcal for cooking, 1,300 to 1,500 Kcal for distillation, 800 to 1,000 Kcal for dehydration, and about 300 Kcal to operate stirring motors and pumps. The drying of by-products from the production of one liter of ethanol might require an additional 600 to 700 Kcal. The production of anhydrous ethanol and dried by-products from grain--representing the high range for process energy--would require 2,800 to 3,800 Kcal/liter. The production of 90 percent ethanol from sugar feedstock without by-product drying--representing the low range for process energy--would require 1,600 to 1,800 Kcal/liter.
The availability and the cost of utilities are critical factors both in the scale and the economics of alcohol production. Two factors have contributed to the failure of ethanol fuel projects in developing countries: (1) plants for the production of ethanol fuel were so large that the support utilities were unable to meet their energy needs; and (2) the plants were sited so far from the feedstock that transportation was not economical.
Plant Scale
Ethanol fuel plants range in size from a few thousand liters to more than 100 million liters of annual production. The design and operation of small-scale plants can be greatly simplified by combining starch hydrolysis, fermentation, and batch distillation in multi-purpose process tanks. The plants could include one or several tanks supplying fermented mash for a single distillation column. Plants up to about 100,000 liters of annual production can be designed this way. Even larger batch plants can be considered if low-cost boiler fuel is available. With good technical assistance, small-scale batch plants can be built and operated with local, community-level resources and skills.
Greater operating efficiency can be obtained in larger plants by separating starch hydrolysis and fermentation in specially designed systems and using continuous feed distillation columns. Generally, the greater capital cost and operating complexity of this type of plant will be returned in operating efficiency. Large-scale alcohol plants require at least some employees with relatively sophisticated management and technical skills. Plant design, equipment, and construction often require resources outside the local community.
COST/ECONOMICS
It is difficult to provide general information about ethanol fuel economics because production costs and product value depend on plant location, feedstock, production scale, and end use.
Ethanol production includes both capital and operating costs. Two important factors in capital costs for small batch plants are starch hydrolysis systems and boiler capacity. In large plants, engineering, distillation systems, and process control are relatively more significant. Generally, capital costs for alcohol plants range from $.50 to $1 (U.S.) per liter of annual production capacity. Based on figures from U.S. plants, capital costs per liter of annual production capacity for very small and very large plants are generally greater than those for intermediate-scale plants--1 to 10 million liters annual production.
The greatest operating cost in ethanol production, regardless of scale, is feedstock. For ethanol fuel production to be profitable, an economical supply of feedstock is essential. In small plants, labor-costs may also be relatively important.
Indirect costs for engine conversion, distribution and marketing, plant utilities, and transportation of feedstock and products are also important in evaluating ethanol production costs.
Ethanol market value depends on end use. The market value of ethanol as a replacement fuel would generally be measured relative to gasoline prices. The market value of ethanol when blended with gasoline may be higher than gasoline because of the increased octane value of ethanol/gasoline blends.
By-product market value is measured against the local price of animal feed. The value is typically determined by comparing the protein content of feeds.
Other factors, aside from ethanol production costs and the market value of ethanol, may also be significant to the economic analysis. Displacement of imported petroleum with domestically produced renewable fuel may improve balance-of-payment deficits and may be economically advantageous despite relatively higher ethanol costs. Opportunities for rural employment, alternative markets for agricultural commodities, and energy independence may provide significant economic advantages in addition to a direct accounting of plant profitability.
IV. COMPARING THE ALTERNATIVES
FUELS COMPETING WITH ETHANOL
Methanol, butanol, and some types of vegetable oil are three alternatives to ethanol. Both methanol and butanol can be used to replace or extend gasoline or diesel fuel. Vegetable oils, however, are limited to replacing only diesel fuel until further research proves otherwise.
Methanol is the most important alternative. It is a liquid alcohol containing one carbon atom ([CH.sub.3]OH). Like ethanol, it is used to replace or be blended with gasoline. Methanol is produced by a chemical process that uses methane as the primary feedstock. Methanol can also be produced from coal or biomass. On a worldwide scale, the methanol production industry is relatively large, and it uses natural gas for feedstock. Methanol production requires high temperature, high pressure, and special catalysts.
This process is much more complex than ethanol production and is generally economical in only very large industrial plants.
Butanol is a four-carbon alcohol. It has two possible chemical structures, depending on the position of the hydroxyl: N butanol ([CH.sub.3] [CH.sub.2] [CH.sub.2][CH.sub.2]OH) and 2 butanol ([CH.sub.3] [OH.sub.1] [CH.sub.2] [CH.sub.3]). Fermentation produces N butanol. Unlike ethanol or methanol, butanol can substitute for or be blended with diesel fuel in compression ignition engines. It is produced by bacterial fermentation of starch- or sugar-containing feedstocks and purified by distillation. The bacteria produce ethanol and acetone in addition to the principal product, butanol.
The production of butanol has two disadvantages: (1) the fermentation of butanol is difficult compared with that of ethanol; and (2) butanol fermentation produces less-useful fuel per unit of feedstock than ethanol fermentation with yeast. Butanol has been produced commercially under wartime conditions. Today, however, butanol is no longer produced commercially for use as fuel.
CURRENT RESEARCH AND DEVELOPMENT
Ethanol fuel production is a well-established commercial technology. But it is also a technology that has room to improve. That is why research and development efforts in ethanol fuel production are ongoing. The research areas relating to this technology that continue to be addressed include (1) feedstock; (2) starch hydrolysis and fermentation process design; (3) ethanol and by-product end uses; and (4) site-specific integration of ethanol production with local agricultural economics.
Feedstock is the most significant cost element in ethanol production. Questions of possible competition for prime agricultural land, and impacts of ethanol production on food supply and distribution are crucial to the social and economic success of this technology. One important area of research is the identification of starch- and/or sugar-containing crops that can be grown on poor land and that require a minimum amount of cultivation and chemical inputs (e.g., fertilizers). Such feedstocks must be compatible with the local climatic conditions, the water resources, and the soil type. They should not disrupt the local agricultural economy. Alternative feedstocks under evaluation in various parts of the world include sago palm, bamboo, sweet potatoes, and honey locust trees. Once potential crops are identified, research will be directed toward increasing yields, adapting crops to specific situations, and developing cultivation, harvest, and storage techniques.
Alternative feedstocks will require research to adapt starch hydrolysis and fermentation equipment and procedures to the particular feedstock characteristics and concentration of fermentable sugars. Fermentation research might also include the selection of yeast strains for improved fermentation efficiency. Improvements could include increased tolerance to high sugar and ethanol concentrations, tolerance to high fermentation temperature, or adaptation to particular feedstock characteristics.
Research needs in ethanol and by-product end uses could include evaluation of technology and economics for uses of ethanol other than as a motor fuel; evaluation of conversion techniques for specific types of engines; and evaluation of specific feedstocks for recovery and use of by-products.
Research on integration of ethanol fuel production with agricultural economies could cover a broad range of topics, including feedstock economics and cultivation, plant and equipment design to fit specific local constraints, process fuel sources, impacts on employment and income distribution, and effects on national balance of payments.
V. INTEGRATION
The successful introduction of ethanol fuel production and use in developing countries requires careful planning. The technology must be integrated with local economic conditions, available resources, and potential end use of both the ethanol and its by-products. The operating efficiency of large-scale ethanol plants may be greater than that of small-scale plants. However, this efficiency may be of little value if the plant is too large for the available feedstock and support utilities or if the local economics of food production and distribution are disrupted.
Ethanol plants should be scaled so that demand for feedstock does not disrupt distribution systems and markets for agricultural commodities. Support utilities and transportation should be able to support the scale of ethanol production. One important, hidden cost of large-scale ethanol plants is the cost of building or upgrading roads, water supply systems, pollution control systems, and electricity generating capacity. The method used to finance these support systems is an important economic question.
Distillers dried grains (DDG) are the major by-product commodity resulting from ethanol production. This high protein product is an excellent livestock feed, and feed lots could be located near the ethanol plant. Another extremely important potential use of this protein-rich material could be as a human food supplement.
End use of the ethanol and by-products must be on a scale that matches production. Technical resources need to be available for engine conversions if necessary. If ethanol is to be blended with gasoline, marketing and distribution systems for ethanol and for ethanol/gasoline blends must be developed in parallel with the construction and operation of ethanol fuel plants.
Proper integration can enhance ethanol production economics and can be achieved with well-designed small- and medium-scale plants. Small-scale plants can often take advantage of low value or waste feedstocks such as food processing waste or damaged or spoiled crops. A variety of low-cost boiler fuels such as biogas, waste heat from other industry or power plants, or biomass can be used if the plants are scaled to match the resources available within economical transport distances. Dehydration can be eliminated if ethanol is used in converted engines. Alternatively, a number of small ethanol plants can supply 80 to 95 percent ethanol to a centralized plant for dehydration and distribution. By-product processing can be reduced if the plant is scaled to supply livestock feed demand in the immediate area of the plant.
Small-scale plants are much simpler to build and operate than large plants. With technical support, small-scale ethanol plants can be built and operated using locally available skills and resources. With the exception of such equipment as motors, boilers, and controls, small-scale plants can be built in any reasonably well-equipped machine shop, provided that technically sound plans are available. Small-scale plants can also be mounted on flat-bed trailers so they can be moved from site to site.
Starch hydrolysis and ethanol dehydration are the two steps requiring long-term purchase of materials outside the local or even national level. The production of starch hydrolysis enzymes and molecular sieves requires relatively sophisticated technology. Enzymes and molecular sieves are supplied by a number of companies. As an alternative to purchasing these materials, they can be manufactured in centralized plants for distribution to small-scale ethanol plants.
VI. CHOOSING THE APPROPRIATE TECHNOLOGY
The decision to produce and use ethanol fuel requires addressing both direct and indirect technical and economic questions. These questions are important on any scale of development ranging from an individual local decision to produce on a small scale to national-level programs.
Direct technical and economic questions in the decision to produce and use ethanol fuel include the cost and the availability of feedstock; ethanol and by-product end uses and marketing; laws and regulations; production scale; and selection of plant design and equipment options.
Factors affecting feedstock availability and cost include transportation, storage, potential spoilage, and seasonal variations in supply and price.
Ethanol and by-product uses are affected by product transportation and distribution, storage, possible spoilage of by-products, seasonal variations in market demand or on-site use, and whether the ethanol is to replace or be blended with gasoline. If ethanol is to be blended with gasoline, the costs and the systems for distribution, blending, and marketing need to be taken into account. If ethanol is to replace gasoline, the costs of engine conversion and limitations to vehicle use are two important factors.
Laws and regulations affecting ethanol fuel production will vary from country to country. Variations may also occur between legal and political jurisdictions within countries. Regulations must be checked for each individual case. The principal regulations are those that prevent the use of fuel ethanol for human consumption. Generally, these regulations require that ethanol be denatured by adding chemical agents to the ethanol to make it unfit for human consumption. The most readily available denaturant for ethanol is gasoline mixed at one percent per volume. Other regulations may govern discharges of liquid and gaseous effluents and occupational safety and health. Laws dictating conformance to building codes (e.g., electric, plumbing, and fire safety codes) may also apply.
Decisions regarding plant scale, equipment, and process design depend primarily on feedstock, the availability of markets for both ethanol and its by-products, and the availability of plant financing. Economies of scale in ethanol fuel production are much less important than well-planned integration of ethanol fuel production with agricultural economics, local transportation, local utilities, and end uses.
Indirect social and economic questions are also very important in the decision to produce and use ethanol fuel. Economic decisions regarding ethanol production may rely more on the ability to meet such objectives as increasing rural employment, achieving energy independence, and providing alternative markets for crops than on direct evaluation of production costs and market values. Technical decisions regarding plant scale, process design, and equipment may be influenced by the ability to meet such objectives as the use of local labor and locally manufactured equipment, the creation of alternative markets for agricultural crops as feedstocks, and the local use of process energy.
The emergence of ethanol as a viable alternative to gasoline has led to two major controversies that can affect decisions regarding ethanol fuel production.
The first controversy concerns the question of net energy yield; that is, whether the energy content of the ethanol is greater than the energy consumed in production. With efficient technology, the energy content of ethanol exceeds the direct in-plant process energy inputs by about 2 to 1. However, one recent analysis, which took into account the energy used to cultivate feedstocks and to transport feedstock and products, calculated that ethanol production consumes more energy than is produced. The technical response to this analysis is that ethanol is not a primary energy source; rather, it is an energy conversion and storage system. In ethanol production, low-quality, diffuse primary energy sources are upgraded to a high-quality, liquid fuel. Solar energy in the form of plant carbohydrate and low-quality boiler fuels is converted to a fuel suitable for use in transportation. In simple terms, the response is that automobiles cannot run on cassava. When ethanol is viewed as an energy conversion system, the net energy question is largely irrelevant. Nevertheless, the question is useful because it points out the need to select those feedstocks requiring relatively little cultivation and low inputs of fertilizer and chemicals, and the need to use low-quality boiler fuels.
The second controversy surrounds the issue of food versus fuel; that is, whether the use of agricultural crops for ethanol fuel production will adversely affect the amount of land available for food production and food supply, as well as affecting food prices. This is a complex question to which there are no absolute answers. On the one hand, a large-scale diversion of food crops to ethanol production could reduce food supplies and increase food prices. On the other hand, a carefully planned and well-integrated ethanol fuel industry does not necessarily result in direct competition for agricultural land and food supplies. Low-value crops grown on marginal land are often good alcohol feedstocks with poor food value. Cultivation of low-value crops may contribute to the economy through conversion to a high-value product. Increased rural employment may increase people's economic access to high-quality food. Ethanol might also be produced from agricultural commodities that would otherwise be exported. Sugar cane, for example, may be worth more as a feedstock for domestic fuel production to displace imported petroleum than as an export crop. The issue of food versus fuel emphasizes the need for careful planning but does not mean that ethanol fuel production is an inappropriate technology.
BIBLIOGRAPHY
The Bioenergy Council. The Bioenergy Directory. Washington, D.C.: The Bioenergy Council.
Bernton, Hal; Kovarik, William, and Sklar, Scott. The Forbidden Fuel: Power Alcohol in the Twentieth Century. New York: Boyd Griffin, Inc., 1982.
Brown, Michael H. Brown's Alcohol Motor Fuel Cookbook. Cornville, Arizona: Desert Publications, 1979.
Carley, Larry W. How To Make Your Own Alcohol Fuels. Blue Ridge Summit, Pennsylvania: Tab Books, Inc., 1980.
Cheremisinoff, Nicholas P. Gasohol For Energy Production. Ann Arbor, Michigan: Ann Arbor Science Publishers, 1979.
De Rasor, Roberto. Alcohol Distiller's Manual for Gasohol and Spirits. San Antonio, Texas: Dona Carolina Distillers, 1980.
Development Planning and Research Associates, Inc. Gasohol: Economic Feasibility Study 1978. Available from the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA 22161.
First InterAmerican Conference on Renewable Sources of Energy. Proceedings of the First InterAmerican Conference on Renewable Sources of Energy, 25-29 November 1979. New Orleans, Louisiana: First InterAmerican Conference on Renewable Sources of Energy, 1980.
Hale, William J. Prosperity Beckons: Dawn of the Alcohol Era. Minneapolis, Minnesota: Rutan Publishing, 1979.
The Mother Earth News. Making Alcohol Fuel. Hendersonville, North Carolina: The Mother Earth News, 1979.
Solar Energy Information Data Bank, Solar Energy Research Institute, U.S. Department of Energy, Alcohol Fuels Bibliography (1901 - March 1980). April 1981, SERI/SP-751-902. This document is available in print from the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402, or in microfiche from the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA 22161.
Solar Energy Information Data Bank, Solar Energy Research Institute, U.S. Department of Energy, Fuel From Farms. A Guide to Small-Scale Ethanol Production. 1980. Also available from the above sources.
U.S. Industrial Chemicals Co. Division of National Distillers and Chemical Corporation. Ethyl Alcohol Handbook. New York, New York: U.S. Industrial Chemicals Co., 1969.
Willkie, Herman F., and Kolachov, Paul J. Food For Thought. Indianapolis, Indiana: Indiana Farm Bureau, Inc., 1942.
Winston, Paul R. Make Alcohol: The New Way To Go. McHenry, Illinois: For-Wins Inc.
The World Bank. Emerging Energy and Chemical Applications of Methanol: Opportunities For Developing Countries. Washington, D. C.: The World Bank. 1982.
Reference inquiries on specific topics relating to ethanol fuel production can be referred through VITA to the staff of Renewable Technologies, Inc., who prepared this report, or to other VITA volunteers with expertise in ethanol fuel.