This article is from ECHO Asia Note #25
David Price is LEAD Asia’s Senior Environmental Consultant and works throughout Asia advising on issues that include environmental sustainability and rural development, climate change, ecological restoration and degraded land remediation, wastewater treatment, erosion control, mangrove restoration, and sustainable practices in agricultural intensification. He and his wife Tammy, members of SIL International, worked as linguists/translators for over 25 years in Papua, Indonesia. David transitioned to his current role during the latter part of that time. David holds a B.Sc. in Zoology and a PGDSc in Conservation Biology. He is currently working on M.Sc. degrees in Zoology and in Environmental Management.
I read with great interest Stuart Brown’s recent article in ECHO Asia Notes #23 entitled The Use of Tropical Forages for Livelihood Improvement in Southeast Asia: A Focus on Livestock (Brown 2015). Mr. Brown is an experienced agricultural consultant most recently working in Cambodia. In the article, he introduced some grass and legume forages (forages are “plant material grazed or fed to livestock”) and recommended them for increased utilization by rural smallholders in parts of Southeast Asia.
As I continued reading, however, I became increasingly uneasy with Mr. Brown’s recommendations. Most of the taxa recommended in the article are serious invasive species, and (I believe) should not be introduced into new areas without extensive evaluations of the possible impacts. In this response article, I will briefly share my experience with invasives, provide an overview of the invasive species issue, show what experts say about the grasses and legumes recommended by Brown, and attempt to offer some guidelines and suggestions for less potentially harmful outcomes.
I am a New Zealander by birth, which impacts my views of invasive species. New Zealand is probably inflicted with a greater number of harmful invasive alien organisms than anywhere else on Earth. Infamously, in their goal to make New Zealand just like ‘the Old Country’ (Britain) and to remedy what they saw as a depauperate native flora and fauna, my country’s founders introduced an impressive range of plants and animals— several deer species from North America, Europe and Asia; chamois and tahr from Eurasia; possums from Australia; peafowl from Asia; and so forth. Rabbits were introduced for hunting and promptly became a major instrument for land degradation and erosion, so we introduced stoats, weasels and ferrets to ‘control’ them (the mustelids found an easier living hunting native birds, driving many to extinction). Introduction of exotic species was not limited to animals; the New Zealand native forests were also quickly converted to pastoral land where introduced species now dominate. Gorse was introduced for hedgerows and Scotch Broom as an ornamental, both of which now cover vast areas in monoculture and which have defied decades of efforts to control them. Over 25,000 plant species were introduced (Duncan & Williams 2002)—compared with New Zealand’s roughly 7,000 native species—and over 2,500 became naturalized in the wild, with more than 300 being classed as invasive species.
My long experience in Indonesia has also allowed me to observe firsthand the introduction of a range of invasive alien species, both plants and animals. My background as an ecologist and naturalist has afforded me some insights into these species’ behavior and impacts, and into the resulting costs and benefits.
Invasive Alien Species
“Invasive alien species are emerging as one of the major threats to sustainable development, on a par with global warming and the destruction of life-support systems.”
Preston and Williams (2003)
Invasive alien species (often IAS in the literature) are those species introduced to an area outside their normal or native range, either purposefully or by accident, whose colonization causes significant harm. The species may become weeds, pests or pathogens, affecting both human interests and natural systems, and impacting agricultural systems, native ecosystems, biological diversity, or human well-being (Perrings et al. 2002; UNEP; CBD). Wellknown examples of invasive alien species include kudzu in the United States, water hyacinth throughout the tropics, zebra mussels in the Great Lakes, and European starlings in North America.
Introduced species are not all bad; in fact, civilization would be impossible without them. Approximately 98% of the U.S. food system, valued at USD 800 billion annually, comes from introduced species such as wheat, rice, corn, and various livestock (Pimentel et al. 2001:1; Pimentel et al. 2005:273). Many naturalized species (non-natives forming sustainable populations without further human facilitations) do not become invasive (Rejmanek 2000:497), and even some that are invasive may ultimately be beneficial. However, a significant number do become harmful invasives. In Europe, 11% of over 10,000 non-native plant populations are thus far known to cause measurable ecological impacts (Vilà et al. 2010).
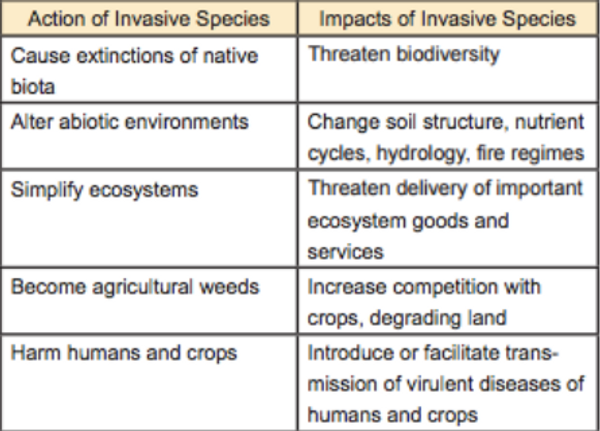
Table 1. Common actions & impacts of invasive species (after Bradshaw et al. 2009)
Alien species invasions are recognized as one of the most significant and pervasive drivers of global environmental change (McNeely et al. 2001; Simberloff et al. 2013) (Table 1). The Millennium Ecosystem Assessment (2005:96-99) lists invasive species as one of the five top drivers of biodiversity loss. In the United States, 42% of the officially recognized threatened or endangered species are at risk primarily due to threats from invasive alien species (Pimentel et al. 2005). Of the almost 700 documented animal species extinctions, over 20% were caused by invasive species (Clavero & García-Berthou 2005). Fifty-six of the world’s 100 most serious invasive species are found in the tropics (ISSG 2007), and Asia is a hotspot. Stephen Elliott of Chiang Mai’s Forest Restoration Research Unit (FORRU) says that one of the biggest hindrances to ecological restoration of tropical forests is invasive species that outcompete and smother native tree seedlings and that modify fire regimes (personal comment).
The socioeconomic costs of invasive species are measured in terms of unemployment, damaged goods/equipment, power failures, food and water shortages, environmental degradation, loss of biodiversity, increased rates and severity of natural disasters, disease epidemics, and lost lives. It is notoriously difficult to assign monetary equivalents to such impacts. However, Pimentel and colleagues (2000) have (conservatively) estimated that “invasion of alien species costs the United States more than USD 100 billion annually,” and over USD 315 billion globally per year (Pimentel et al. 2001). Globally, agricultural losses are estimated to be between USD 55 and 250 billion a year (Bright 1999). Even single species can be responsible for losses running in the millions of dollars. The Latin American golden apple snail, Pomacea canaliculata, was introduced into the Philippines in the 1980s to provide a ‘high protein food source’ and has subsequently caused losses to rice crops in the order of USD 1 billion annually (Naylor 1996). Mainland China currently has at least 400 invasive species which cost the country an estimated USD 14.5 billion annually (Agoramoorthy & Hsu 2007).
Some large-scale, far-reaching impacts of invasion are not readily detectable, such as the multiple impacts by introduced nitrogen-fixing plants on ecosystem functions (Vitousek et al. 1987). Ecosystems may be modified below- and above-ground by introduced plants that transform ecosystem structure and function, especially through community composition and altered nutrient cycling (Simberloff et al. 2013). Soil chemistry, hydrology, and fire regimes can be altered (Cronk & Fuller 1995). Erosion regimes can be changed and physical structures (e.g. dunes) can be added (Simberloff 2011). A common impact is general land degradation, one of the foremost drivers of poverty (Kaimowitz & Sheil 2007).
The consequences of invasion may take years or decades to be identified, and invading plant species may not ‘break out’ until many years after naturalization (Essl et al. 2011). In Florida, Brazilian pepper remained very restricted in range for a century, but then rapidly expanded across a wide area (Crooks 2011). Some problematic plants introduced to Europe have taken between 150 and 400 years to reach their fullest areal extent (Gassó et al. 2010), underscoring the extent to which humans do not know the consequences of species introductions.
The invasives problem is so great and universal in extent that we have even coined a term for its inevitable (without our intervention) outcome. ‘Homogenization’ is the process in which ecological communities and ecosystems become increasingly dominated by a small number of widespread, human-adapted species (Millennium Ecosystem Assessment 2005:79.) Homogenization describes the way such invasions and introductions are transforming ecosystems to simpler, less productive states and diverse communities to simple ones with vast numbers of few species. The end results are novel ecosystems which provide fewer of the goods and services that humans need to live and thrive. This is now rapidly occurring in every place on Earth.
“The litany of negative, far-reaching impacts of invasions suggests that proposed introductions warrant great caution.”
(Simberloff et al. 2013)
What gives a particular species the propensity towards invasiveness? Invasive species have characteristics or traits that give them significant competitive advantage over existing native species, or increased ability to colonize marginal, disturbed habitats. These traits include the ability to reproduce, grow and disperse rapidly; aggressive competition for resources such as water, nutrients, and space; and lack of natural enemies in their new environment. Invasive species are often pioneer species, and tend to be generalists in terms of requirements.
Connections with Fodder Crops
The goal of developing new pasture plants is the sustainable intensification of agriculture. Invasive species are a serious barrier to this goal because they increase the environmental and economic costs of food production (Driscoll & Catford 2014). Enormous effort is put into developing new varieties that will facilitate the goal, but agriculturalists and extension workers invest very little thought and even less money in order to determine invasion risk (Driscoll et al. 2014). Environmental risk assessments are rarely carried out, partly because the corporations and organizations that develop them bear no legal or financial responsibility for the costs when such plants become invasive burdens (Driscoll et al. 2014).
Most research conducted on the invasion risk of new pasture plants is carried out by the environmental and conservation science communities. The findings are stunning—new pasture crops show an overwhelming propensity to become seriously invasive. Over 90% of new pasture species developed by agribusiness become invasive weeds (Driscoll & Catford 2014). The characteristics that are selected for—fast growth, efficient reproduction and dispersal, and tolerance of broad environmental conditions—are the very traits that make such plants invasive (ibid). Processes like hybridization and allopolyploidy (how we got wheat) increase an organism’s genetic diversity and enhance its capacity to flourish across a broad range of conditions (Driscoll et al. 2014). New crop species may interbreed with existing weed species, intensifying invasive tendencies. Invasiveness is often recognized as an important trait in successful novel pasture crops—they should be able to survive and spread unassisted (Miller et al. 1997).
Review of Forage Crops Recommended by Brown
Many useful online resources provide information on various species that are known or suspected to be invasive in different countries. At least one site rates plants for risk: less than 1 = low risk, free to import; greater than 6 = high risk, reject; between 1 and 6 = requires further evaluation, proceed with caution.
• Global Invasive Species Database (GISD) http://www.issg.org/database/welcome/
• Pacific Island Ecosystems at Risk (PIER) http://www.hear.org/pier/index.html
• CABI Invasive Species Compendium (CABI) http://www.cabi.org/isc/
• IUCN Species Survival Commission Invasive Species Specialist Group http://www.issg.org/
• Island Biodiversity and Invasive Species http://ibis.fos.auckland.ac.nz/
• Tropical Forages (TF) http://www.tropicalforages.info/index.htm also lists possible invasion tendencies
The following are brief statements regarding the invasiveness or potential invasiveness of the species listed in Brown’s article. Where taxonomy differs or is in confusion I have deferred to the Integrated Taxonomic Information System (http://www.itis.gov/) as the authority.
Megathyrsus maximus (syn. Urochloa maxima, Panicum maximum): Guinea Grass
GISD: “... has become prevalent in Samoa and Tonga ... a problem species in Guam and Hawaii ... can form dense stands and displace native species ... forms dense stands in open pastures and disturbed areas … can suppress or displace local plants on fertile soils in pastures ... resistance to drought also means it builds up a dangerous mass of plant material so when fires occur, the blaze is fiercer and native plants which have not built up fire-tolerance are wiped out ... can survive fires [so] can dominate the ground after a fire ... can tolerate brackish water and interfere with stream flow due to its highly aggressive invasive habit.”
PIER: gives the species a 6, meaning ‘high risk’ and ‘reject.’ “A serious weed in tropical and subtropical crops and wastelands. Very common in open disturbed areas of forests, wastelands, and roadsides...in mesic to humid lowlands. Grows into tall, dense stands, displacing natives, a fire hazard in dry periods. In Hawaii, naturalized and common, 0-850 m ... in Fiji, a weed of sugarcane fields, roadsides, and river banks ... in Australia, ... forms dense stands that may exclude some native species, particularly some early flowering grasses ... in New Caledonia, now widely dispersed.”
CABI: “a highly successful invader in tropical and warm temperate areas after introduction as fodder. It can spread from seed, is highly competitive with native flora, and while it is highly fire resistant it can quickly spread to invade gaps left in natural vegetation after fire.”
TF: “a very effective colonizer in ungrazed areas, particularly where some form of soil disturbance has occurred ... spreads along water courses and ungrazed roadsides, and has been listed as a weed in many countries ... a major weed in sugarcane fields, due to its ability to grow under shaded conditions. ...”
Brachiaria species hybrid (cv. Mulato II; Cayman)
Closely related to the above species. I am unable to find invasive information on this taxa, however, from the TF site: “Likely to be similar to B. brizantha [a synonym of Urochloa brizantha], having potential to colonize disturbed areas.” PIER gives the genus rating 4—requires further evaluation.
Paspalum atratum
At least three other Paspalum species have significant impact as invasive species and are listed as noxious weeds somewhere. There appears to be taxonomic confusion between this species and P. plicatulum, a low-risk invasive species, at least in New Caledonia and Cuba. Another, P. paspaloides or knotgrass, is invasive in Europe (DAISIE 2009). Caution is warranted. Vetiver grass (Chrysopogon zizanioides), a non-invasive native of India, should be considered a superior alternative on the grounds that it is sterile and less competitive with native plants. Both have similar forage values and limitations (only young leaves are palatable), but vetiver grass has many additional characteristics that make it useful for addressing a wide range of agricultural and sustainability issues.
Pennisetum purpureum: Elephant Grass, or Napier Grass
This species is classed as ‘invasive’ in so many countries that it should NOT be promoted in any way. It may become one of the most serious weeds that Southeast Asia will have to deal with during the next thirty years or so. PIER gives it extremely high ratings for invasiveness and risk.
PIER: “A major problem in the Galápagos Islands. One of the most invasive weeds in Papua New Guinea ... subject to an eradication program on Mangaia ... planting of this species prohibited in Miami-Dade County, Florida (U.S.) ... Not-withstanding its value as forage, elephant grass has become one of the worst weeds in the tropics because of the difficulty of controlling it in croplands and fallow areas.”
CABI: ... “P. purpureum is considered one of the most successful invasive grasses in the world. ... included in the Global Compendium of Weeds where it is listed as an agricultural and environmental weed as well as an invasive species ... an aggressive grass that grows rapidly, colonizing new areas and forming dense thickets. Once established, it can change features of ecosystem functions by altering fire regimes, hydrology cycles, biophysical dynamics, nutrient cycles, and community composition ... well adapted to drought conditions and can also dominate fire-adapted grassland communities ... has the capability to resprout easily from small rhizomes left after disturbance, resulting in the out-competing and smothering of native plant communities.”
In Brown’s paper, an editors’ note mentioned a hybrid. I strongly recommend that ECHO rigorously evaluate it for invasibility and control before considering release [Eds’ Note: The developer asserts it is a non-GMO sterile hybrid cross]. Also, either this species or a close relative (P. setaceum) is being promoted in Thailand and the Philippines (and probably other parts of Asia), though mostly as an ornamental—it is a spectacularly pretty addition to rock gardens. In some places it is promoted under the misnomer ‘Purple Vetiver.’
Stylosanthes guianensis: Common Stylo
Stylosanthes guianensis appears to be highly invasive almost everywhere it has been introduced. PIER gives it high invasiveness and high risk ratings, and recommends rejecting it for importation. In Australia, common stylo is a weed of open woodlands, grasslands, floodplains, levee banks, roadsides, disturbed sites, waste areas and crops in tropical and sub-tropical regions. The plants are considered invasive and environmental weeds in Taiwan (Shan-Hua Wu et al. 2003), Pacific Islands (PIER), and Hawai’i (Chakraborty 2004). Some Stylosanthes species, in particular S. guianensis, have been deemed a conservation threat because they are too aggressive and easily invade areas outside pastures in Australia (Maass & Sawkins 2004). Stylosanthes can dominate pasture, causing long-term effects such as major rises in soil acidity, a decline in biodiversity and increased risk of soil erosion (Jones et al. 1997). Other detrimental effects include loss of soil surface stability, nutrient depletion and vegetation changes, including weed invasion (Maass & Sawkins 2004:59).
Arachis pintoi: Pinto Peanut
Yay, finally! This species does not appear to be invasive in the least. PIER gives it a -1 rating, safe as safe can be. It has many benefits, as Brown mentions, but also provides a fast-growing groundcover that can protect soil against erosion caused by destructive raindrops. Promote this crop!
Leucaena leucocephala
Leucaena leucocephala was a mainstay of the Green Revolution. The editor already correctly noted in Brown’s article that Leucaena leucocephala can become a serious invasive pest in some countries. It can sometimes spread to become a troublesome weed, resulting in a monoculture (McNeely & Scherr, 2003:81).
PIER: Gives it a ‘high risk’ and a ‘Reject’ score. “forms extensive and dense thickets displacing the original vegetation and reducing species richness ... forms dense thickets, excluding all plants ... grown for fodder, but unless severely grazed or controlled, it spreads rampantly throughout adjacent areas ... in Hawai‘i, naturalized and very common, sometimes forming the dominant element of the vegetation, in low elevation, dry, disturbed habitats ...”
CABI: “an aggressive colonizer of ruderal sites and secondary or disturbed vegetation ... declared a category 2 weed in South Africa ... listed as invasive species on Puerto Rico, one of the most problematic invasives on the island ... impacts include reduction in land area for activities such as farming when the species becomes weedy on abandoned cultivated land or pasture ... possible allelopathic effects ... outcompetes other vegetation, resulting in reduction of species diversity ... a potential habitat transformer ... degrading native forests in Hawai’i ... a number of examples of where monospecific thickets of L. leucocephala are degrading the indigenous flora ... in Ghana it is competing with rare endemic species ... introduced to Guam to reforest bombed areas, but now preventing the establishment of indigenous species ... preventing the regeneration of native forest vegetation in Mauritius ... while highly useful as a fodder plant, it is toxic to livestock if it is used in too great a quantity in the diet.”
GISD: “listed as one of the ‘100 of the World’s Worst Invasive Alien Species’ ... can form dense monospecific thickets and is difficult to eradicate once established ... renders extensive areas unusable and inaccessible and threatens native plants ... not known to invade undisturbed closed forest habitats ... reported as a weed in >20 countries across all continents except Europe and Antarctica ... a weed of open, often coastal or riverine habitats, semi-natural, and other disturbed or ruderal sites and occasionally in agricultural land ... can form dense monospecific thickets which are reported to be replacing native forest in some areas and threatening endemic species of conservation concern in some areas ... can render extensive areas of disturbed ground unusable and inaccessible.”
Gliricidia sepium
Not listed as invasive by GISD. This species is extremely useful as a nurse plant for native seedlings in tropical forest restoration and is extensively used in agroforestry.
PIER: “Low invasion risk ... can grow into monospecific stands” [I’ve never seen it do that.]
CABI: “a moderate or potentially invasive species ... an adaptable, fast growing tree, with the ability to disperse seeds up to 40 m from the parent tree from exploding pods ... a colonizer of disturbed ground ... has become a weed in Jamaica ... regarded as a potential weed in Australia.”
Where do we go from here?
Though somewhat of a cliché, it’s true that life is a series of trade-offs or compromises. Potentially invasive forage crops are no exception. In many situations, the benefits of introducing a potentially invasive species greatly outweigh the costs; perhaps many of Brown’s readers live in such contexts. In places where rural agricultural development takes place, many (if not most) of these invasive species may be already established but underutilized. Promoting their use might control their spread into undesirable places. On the other hand, often native analogs can be found that offer similar benefits to potentially invasive species, yet the native plants have been overlooked, probably because of our almost universal bias towards exotic species when utility is the chief consideration.
When considering whether to introduce or reintroduce any organism (not just forage crops), several considerations should be taken into account. What is the organism’s track record elsewhere—is it known to be invasive? If so, how risky is it and how is it managed (Hulme 2012)? NGOs with resources such as ECHO should be carrying out extensive weed assessment studies before promoting suspect crops. Many such risk assessment frameworks are available, such as in Driscoll et al. (2014:16625), and can be adapted to specific contexts.
National biosecurity has proven extremely successful and cost-effective in managing novel invasive species introductions in countries that take it seriously, such as New Zealand and Australia (Springborn et al. 2011), although since many invasive species have already colonized, it is perhaps a case of too little too late. In fact, stringent biosecurity can bring huge economic benefits (Simberloff et al. 2013:61; Keller et al. 2007). But many of us work in countries with inadequate or poorly implemented biosecurity frameworks, where regulations covering invasive species are not enforced on the ground, in the villages, and on the farms. In such cases, a culture of “every man doing what is right in his own sight” seems to reign. Some argue, “I will put the needs of the communities over the protection of the environment,” but this is a patently misleading and self-defeating argument since such a dichotomy does not exist—what is bad for the environment will ultimately be bad for communities living in that environment.
Where an action has a suspected risk of causing harm to humans or the environment, and in the absence of a scientific consensus, the Precautionary Principle places the burden of proof (that an action or policy is not harmful) on those taking the action. Those (including us) who would undertake risky initiatives must bear the responsibility for ensuring that they will not cause harm.
“When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically.”
Wingspread Statement on the Precautionary Principle, Jan. 1998
Currently, the public bears the cost of environmental weeds that have escaped from pastures (Driscoll et al. 2014). The agribusiness industry continues to create new plants, promote and release them, with little thought of negative consequences and with no legal or financial culpability. Driscoll and Catford (2014) urge governments to include potential environmental damage when screening new pasture varieties, and to introduce a ‘polluter pays’ penalty system. Though it is a great idea, I don’t see it happening anytime soon—there are powerful, international, vested interests in agribusiness.
Before we decide whether or not to promote or release a potentially invasive species, and after first doing a risk assessment, we development and extension workers might do well to ask ourselves one question: “Would I be willing to be legally responsible for the costs incurred by the people of this nation if this species turns out to be invasive?” Personally, I think agribusiness, NGOs, and development workers who take such risk upon themselves should be held legally responsible in the case of invasive outbreaks, and financially so for agribusiness.
Let’s consider native and local alternatives that might offer similar benefits with reduced risk. The ECHO Asia Seed Bank is already trying to do this . For example, non-invasive vetiver grass (Chrysopogon zizanioides) has moderate potential as a fodder crop but carries none of the risks mentioned above. Several closely related species can be found in Africa, Thailand and elsewhere. Though fertile and potentially invasive outside of their original distribution, they are being used within their normal range effectively and safely in several initiatives (for example C. nigricans in Ghana and C. nemoralis in Thailand). Another example is the use of Indonesian albizia (Paraserianthes falcataria) within its natural distribution of Eastern Indonesia and Papua New Guinea. Officially the fastest-growing tree in the world, this species tends to become somewhat invasive when introduced to new areas (like the Philippines), but is an excellent alternative to Leucaena leucocephala in its natural range. Extension workers are perfectly positioned to work with local indigenous people to identify such native analogs of potentially harmful exotic invasives.
Finally, I would like to point out that even when a plant has become a significant environmental, social and/or economic burden, there is still hope. Eradication is often possible! Despite widespread belief to the contrary, eradication technologies have improved to the point where eradication attempts are feasible. Genovesi (2011) reviewed more than 1,000 attempted eradications, including of some long-standing invasions, finding that 86% of these had succeeded. The benefits of eradication can be enormous. Allan and colleagues (2010) found that eradication of invasive honeysuckle drastically reduced the risk of tick-borne Lyme Disease in the United States, stating “management of biological invasions may help ameliorate the burden of vector-borne diseases on human health.” Eradication, where possible, can be far cheaper than long-term management of invasives. Early extirpation of introduced plants in New Zealand costs on average 40 times less than later attempts (Simberloff et al. 2013:61). Eradication, especially using ecological restoration techniques, can restore ecosystem services that have been lost to an invasion.
In conclusion, while we cannot and should not encourage bans on all invasive species, some certainly ought to be banned in the places where we work. At the very least, we should proceed with a full and informed perspective. I don’t want to shame anyone, but I want to call for a measure of responsibility and wise consideration about how we use specific forage crops and other plant species that could be invasive. As community development workers, we must consider our responsibility as we think about introducing species that many cause potential long-term suffering. Let us not carry on blithely, recommending a suite of plants that offer some benefits, without at least strongly cautioning of their potential disastrous impacts. Otherwise we will jeopardize the very goal we strive for, that is included in Brown’s title: livelihood improvement.
[Author’s Note: Though I primarily consult for LEAD Asia and its partners, I am happy to help others with any environmental and development issues, particularly in developing nations and in Asia. I can be contacted at anura@wbt.org.]
Appendix
[Note from the Eds.’: Below is a follow-up warning and helpful information from the ECHO Seed Bank regarding plant introductions:]
The Nature of Plant Introduction: Some Important Cautions
ECHO supplies small seed packets for trial. It is important to understand that the plants must be treated at first as experimental before making recommendations to members of your community. Many, many development workers have introduced and promoted ‘miracle technologies’ and ‘wonder plants’ before giving them adequate trial and experimentation on-site. Not even studies in the same country can guarantee acceptance or success. Hasty introductions of new ideas or plants are likely to encounter serious problems. Farmers may have planted their fields with the new varieties or invested their savings in the new tool when the problems surface; perhaps a pest or disease strikes, or the equipment is faulty or unsuitable. In the end, farming families will suffer, and the development worker will understandably have a very difficult time promoting any further ideas or innovations. People may lose confidence or trust, with serious consequences for your work or ministry.
There are many advantages to conducting your own trials before disseminating seeds in the wider community. It is important to know whether the plant can grow in your area before farmers devote land and time to cultivating it. Through conducting trials you may find the best ‘window’ in your seasons for the optimal performance. You receive only a small packet of seeds from ECHO; if the plants produce well, you will have plenty of seeds to share. If the plants do not grow and produce seed, perhaps they are not suited to your region. Should the species be enthusiastically accepted, ECHO can refer you to commercial sources for some seeds if you need larger quantities or want to broaden the genetic base. If the plant holds great promise in your area, it is best to obtain more seeds from another source before the planting areas become too large. Genetic diversity not only offers potential for superior plants to be identified, but also affords protection in case of disease outbreak.
Beyond avoiding the risk of total planting failure, small trials allow you to evaluate the ‘weed potential’ of certain species in your area. Watch the planting carefully the first few seasons to make sure it is not likely to become a problem plant. Unfortunately, one definition of a weed, “plants which thrive under stressed conditions, often with high seed production,” fits some of the plants in ECHO’s seedbank. We are very aware of this risk and have in fact eliminated certain species from our seedbank when the danger of introducing a weed seemed too great. However, hardy plants which can establish themselves may be a great blessing in many situations; for example, it is difficult to imagine a tree which could become a pest in certain areas of Africa or Haiti with severe fuelwood shortages. Sending out only small trial packets of seed is another safeguard against introducing a weed, as too-aggressive plants may be identified and controlled easily in a small area. Finally, remember that the plants in ECHO’s seedbank are commonly accepted food plants somewhere in the world, even if very localized. In this, too, there is a measure of safety as we can all learn and benefit from the years of plant selection by people in other parts of the world. In all cases, we look upon those who request seed as collaborators with us in field trials. This does not mean that you must do elaborate experimentation, but we do expect you to take time to write to us after the food has been harvested, letting us know your general impressions on its suitability to the region and the culture. A seed trial report form (in English, French, or Spanish) is sent along with your seeds. We enter your results in our database and use this information to make more refined recommendations to others and to share with interested scientists. These reports are very important to us, to be aware of germination or weediness problems, as well as to learn of successful introductions and acceptance of the plant in the community. We are always glad to receive the seed trial reports, but we also have special interest in longer-term results of plant introductions and the effects of ECHO’s work. If you receive seed from ECHO and the plants are adopted in the fields and gardens in your area, please let us know.
References
Agoramoorthy, Govindasamy & Hsu, Minna J. 2007. Ritual releasing of wild animals threatens island ecology. Human Ecology, 35(2): 251-254.
Allan, Brian F., Dutra, Humberto P., Goessling, Lisa S., Barnett, Kirk, Chase, Jonathan M., Marquis, Robert J., Pang, Genevieve, Storch, Gregory A., Thach, Robert E. & Orrock, John L. 2010. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proceedings of the National Academy of Sciences, 107(43): 18523- 18527.
Bradshaw, Corey J.A., Sodhi, Navjot S. & Brook, Barry W. 2009. Tropical turmoil: a biodiversity tragedy in progress. Frontiers in Ecology and the Environment, 7(2): 79-87.
Bright, C. 1999. Invasive species: pathogens of globalization. Forest Policy, 1999: 51–64.
Brown, Stuart. 2015. The use of tropical forages for livelihood improvement in Southeast Asia: A focus on Livestock. ECHO Asia Notes, 23: 3-9.
CBD. n.d. Invasive Alien Species. Accessed 3 July 2015 from https://www.cbd.int/invasive/
Chakraborty, S. (ed.) High-yielding anthracnose resistant Stylosanthes for agricultural systems. ACIAR Monograph, 111, 268 p.
Clavero, Miguel & García-Berthou, Emili. 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology and Evolution, 20(3): 110.
Cronk, Q.C.B. & Fuller, J. 1995. Plant invaders: the threat to natural ecosystems. London, UK: Chapman & Hall and World Wide Fund for Nature.
Crooks, J.A. 2011. Lag times. In Encyclopedia of Biological Invasions (Simberloff, D. & Rejmánek, M., eds), pp. 404–410, University of California Press.
DAISIE. 2009. A Handbook of Alien Species in Europe. Springer: Berlin.
Driscoll, Don A. & Catford, Jane. 2014. New pasture plants pose weed risk. Nature, 516(7529): 37.
Driscoll, Don A., Catford, Jane A., Barney, Jacob N., Hulme, Philip E., Inerjit, Martin, Tara G., Pauchard, Aníbal, Pysek, Petr, Richardson, David M., Riley, Sophie & Visserm, Vernon. 2014. New pasture plants intensify invasive species risk. Proceedings of the National Academy of Sciences, 111(46): 16622-16627.
Duncan, R.P. & Williams, P.A. 2002. Darwin’s naturalization hypothesis challenged. Nature, 417: 608-609.
Essl, Fanz, Dullinger, Stefan, Rabitsch, Wolfgang, Hulme, Philip E., Hülber, Karl, Jarosík, Vojtech, Kleinbauer, Ingrid, Krausmann, Fridolin, Kühn, Ingolf, Nentwig, W., Vilà, M., Genovesi, P., Gherardi, F., Desprez-Loustau, M.-L., Roques, A. & Pysek, P. 2011. Socioeconomic legacy yields an invasion debt. Proceedings of the National Academy of Sciences, 108(1): 203-207.
Genovesi, P. 2011. Are we turning the tide? Eradications in times of crisis: how the global community is responding to biological invasions. In Island Invasives: Eradication and Management (Veitch, C.R. et al., eds), pp. 5–8, IUCN.
Gassó, Nuria, Pyšek, Petr, Vilà, Montserrat & Williamsson, Mark. 2010. Spreading to a limit: the time required for a neophyte to reach its maximum age. Diversity & Distributions, 16(2), 310-311.
ISSG (Invasive Species Specialist Group). 2007. Global invasive species database. Auckland, New Zealand: World Conservation Union.
Jones, P.G., Galwey, N.W., Beebe, S.E. & Tohme, J. 1997. The use of geographical information systems in biodiversity exploration and conservation. Biodiversity and Conservation, 6: 947-958.
Hulme, P.E. 2012. Weed risk assessment: A way forward or a waste of time? Journal of Applied Ecology, 49(1): 10-19.
Kaimowitz, David & Sheil, Douglas. 2007. Conserving what and for whom? Why conservation should help meet basic human needs in the tropics. Biotropica, 39(5): 567-574.
Keller, Reuben P., Lodge, David M. & Finnoff, David C. 2007. Risk assessment for invasive species produces net bioeconomic benefits. Proceedings of the National Academy of Sciences, 104(1):203-207.
Maass, Brigitte L. & Sawkins, Mark. 2004. History, relationships and diversity among Stylosanthes species of commercial significance. Pp 9-26 in Chakraborty, S. (ed.) High-yielding anthracnose resistant Stylosanthes for agricultural systems. ACIAR Monograph, 111, 268 pp.
McNeely, Jeffery A. 2001. Invasive species: a costly catastrophe for native biodiversity. Land Use and Water Resources Research, 1(2): 1-10.
McNeely, Jeffrey A., Mooney, H A., Neville, L.E., Schei, P.J. & Waage, J.K. (eds.). 2001. Global Strategy on Invasive Alien Species. IUCN, Cambridge.
McNeely, Jeffery A. & Scherr, Sara J. 2003. Ecoagriculture: Strategies to feed the world and save wild biodiversity. Island Press: Washington, D.C.
Millennium Ecosystem Assessment. 2005. Ecosystems and Human Well-being. Island Press: Washington, DC.
Miller, C.P., Rains, J.P., Shaw, K.A. & Middleton, C.H. 1997. Commercial development of Stylosanthes. II. Stylosanthes in the northern Australian beef industry. Tropical Grasslands, 31: 509-514.
Naylor, Rosamond L. 1996. Invasions in agriculture: Assessing the cost of the Golden Apple Snail in Asia. Ambio, 25(7): 443-448.
Perrings, Charles, Williamson, Mark, Barbier, Edward B., Delfino, Donriana, Dalmazzone, Silvana, Shogren, Jason, Simmons, Peter & Watkinson, Andrew. 2002. Biological invasion risks and the public good: an economic perspective, Conservation Ecology, 6(1): 1.
Pimentel, David, Loch, Lori, Zuniga, Rodolfo & Morrison, Doug. 2000. Environmental and economic costs of non-indigenous species in the United States. BioScience, 50(1): 53-65.
Pimentel, David, McNair, S., Janecka, J., Wightman, J., Simmonds, C., O’Connell, C., Wong, E., Russel, L., Zern, J., Aquino, T. & Tsomondo, T. 2001. Economic and environmental threats of alien plant, animal, and microbe invasions. Agriculture, Ecosystems & Environment, 84(1): 1-20.
Pimentel, David, Zuniga, Rodolfo & Morrison, Doug. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics, 52: 273-288.
Preston, G.& Williams, L. 2003. Case Study:The Working for Water Programme: Threats and Successes. Service Delivery Review, 2(2): 66-69.
Rejmanek, Marcel. 2000. Invasive plants: approaches and predictions. Austral Ecology, 25(5): 497-506.
Shan-Hua Wu, Shu-Miaw, Chaw & Rejmanek, M. 2003. Naturalized Fabaceae (Leguminosae) species in Taiwan: the first approximation. Botanical Bulletin of Academia Sinica, 44: 59-66.
Simberloff, Daniel. 2011. How common are invasion-induced ecosystem impacts? Biological Invasions, 13(5): 1255-1268.
Simberloff, Daniel, Martin, Jean-Louis, Genovesi, Piero, Maris, Virginie, Wardle, David A., Aronson, James, Courchamp, Franck, Galil, Bella, García-Berthou, Emili, Pascal, Michel, Pylet, Petr, Sousa, Ronaldo, Tabacchi, Eric & Vilà, Montserrat. 2013. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology and Evolution, 28(1): 58-66.
Springborn, Michael R., Romagosa, Christina M. & Keller, Reuben P. 2011. The value of nonindigenous species risk assessment in international trade. Ecological Economics, 70(11): 2145-2153.
UNEP. n.d. Invasive alien species: a growing threat in regional seas. Accessed 3 July 2015 from http://www.unep.org/ regionalseas/publications/brochures/pdfs/ invasive_alien_brochure.pdf
Vilà, Montserrat, Basnou, Corina, Pyšek, Petr, Josefsson, Melanie, Genovesi, Piero, Gollasch, Stephan, Nentwig, Wolfgang, Olenin, Sergei, Roqyes, Alain, Roy, David, Hulme, Philip E. & DAISEI partners. 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment, 8(3): 135-144.
Vitousek, P.M., L.R. Walker, L.D. Whiteaker, D. Mueller-Dombois, & P.A. Matson. 1987. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science, 238(4828): 802-804.