This article is from ECHO Asia Note #28
[Eds.’ Note: Ken is a missionary from Church Mission Society, Australia. He and his family have lived and worked in Mondulkiri province, Cambodia, for the past eleven years. Ken has worked on various agricultural and animal health issues facing indigenous communities in that province. For the past five years, he has been working with the local KEC churches (Khmer Evangelical Church) to establish the Ntuk Nti Center, a small farm resource center on the church’s land near Sen Monorum town, Mondulkiri.]
Introduction
For both farmers and researchers in the tropics, seed saving can be very frustrating. In Mondulkiri province, farmers are rarely able to keep seed for more than the six months between harvest and the new planting season. Seeds stored longer than this tend to either pick up moisture from the extra humid air during the wet season and lose viability, or suffer from insect pests that proliferate and destroy the seed. At our resource center, we had wanted to build up a seed inventory of many useful plant species without having to grow out each variety every year. However, similar to the farmers, our seed had often quickly lost viability or was destroyed by pests while stored.
Refrigeration and freezing of most orthodox seeds are well known methods for extending seed life, (See ECHO Asia Note 14 “Vacuum Sealing versus Refrigeration”), but don’t offer an appropriate solution in areas like Mondulkiri province, where electricity, if available, is unreliable and expensive. In partnership with ECHO Asia and with funding from the Presbyterian Hunger Program, staff members at Ntuk Nti have been conducting research over the past year to design and test appropriate options for seed saving. In this article we share some of our findings - useful methods to improve seed storage without electricity that even the poorest and most isolated farmers can use.
Drying
To keep seeds for more than a year, they need to have a moisture content (MC) of 10% or less. This requires thorough drying before storage. While many books and articles warn against drying seeds in direct sunlight, there may be no other option for resource-poor farmers in the tropics. Drying thin layers of seed in the shade will reduce their moisture content, but when the daytime relative humidity (RH) is above 60%, seed of many species will not dry sufficiently in the shade to meet proper storage requirements. Our measurements show that in Mondulkiri province, periods with an RH of 60% or less only occur in the driest part of the year and then only briefly during the middle of the day. Freshly harvested seed that is dried in the shade rarely becomes dry enough for long-term storage. On the other hand, we have successfully dried seed in the sun to 9% MC even during the rainy season! This was achieved by placing the seed in shallow trays in direct sun on three to four selected sunny days. Between these selected daytime exposures, the seed was kept in sealed containers to prevent re-absorption of moisture from the humid air during the night and early morning, when relative humidity tends to be the highest (often RH of >90%).
Often in literature, we are warned against drying seeds in the sun at temperatures exceeding 36°C. However, at Ntuk Nti we recorded temperatures well over 50°C in several species of cereal and vegetable seeds that were drying in the sun, with no resulting reduction in their germination rates. This may be species-dependent, so we recommend caution with vegetable seeds. We think that the speed of drying may contribute more to damage than high temperatures. Speed of drying is affected by the temperature, air movement, and RH during the drying process. Seeds in the summer sun in temperate climates would dry very rapidly, causing damage, while in the tropics the high relative humidity limits the rate of drying. At Ntuk Nti, it has been our practice to take freshly harvested seeds (often about 16% MC or more) and dry them in the shade for a few days. This allows the seeds to dry down slowly, avoiding cell damage. Then we finish drying in the sun to get the seeds to 10% MC or below (even 7% in many species) without harming them. We used a moisture meter for our research, but farmers can tell when 10% MC is achieved because the seeds will shatter when hit with a hammer. For further information on seed drying, refer to EDN 109, and to “Seed Moisture and Drying Principles,” (Danida Forest Seed Centre, Stubsgaard F, & Poulsen K (1995)).
Containers
Once you have sufficiently dried seeds, you need to seal them so that humid air will not cause seeds to reabsorb moisture. Originally, we used containers, such as powered milk or milo tins for larger quantities of seed, and small plastic herb containers or pill bottles for small quantities. However, these didn’t seal out the moist air sufficiently! Jam or pickle jars that have a clean rubberized seal inside the lid work much better, and will seal very well if the lid is heated just before putting it on. Zip lock bags are another possibility, but unless these are then put inside a tin with a close-fitting lid, they will be damaged by cockroaches, ants, or mice and seeds will then be exposed to moist air. Even when bags are placed in protective containers, it only takes a few weevils in the seed to chew a hole in a zip lock bag!
In our rural community, none of the above containers are commonly available. Wanting to find more appropriate options, we have been experimenting with glass sauce and beer bottles for smaller amounts of seed, and large plastic jerry cans for large amounts of seed. The former can be obtained for free in large numbers from rubbish dumps and can be sealed with a rubber stopper or a small disc of rubber inner tube held in place with electrician’s tape. The jerry cans are cheaply and readily available. When new, the jerry cans have a seal that works well. Seal older cans by placing a bi-folded plastic bag over the opening and screwing the lid tightly down on it. Both bottles and jerry cans have narrow openings that require a funnel for filling with seeds, but they have the advantage of being easy to seal. Also, when these containers are opened to remove some seeds, the smaller opening limits exposure to outside humid air. We have stored bulk rice and beans in these jerry cans for two years at ambient room temperature with no reduction in germination rate.
All containers need to be kept out of direct sunlight and in as cool a location as possible in order to maximize storage life and maintain high germination rates and seed health for planting.
Oxygen Reduction
Once dry seed has been placed in a suitable sealed container, seed should last three or more years with good viability. However, there is still the possibility that weevils or other insects could be in the seed. Left alone, these will multiply. The insects may eat the seeds and/or their respiration may produce moisture that will eventually cause all the seed to rot. We have found that insects are less of a problem when seed is harvested at the right time, quickly dried, and then immediately stored in full, sealed containers. But if there are delays in seed processing or seeds are obtained from other sources, seeds may already be contaminated by insects. To ensure that these seeds do not rot in storage, we must reduce the available oxygen to the insects so they are unable to breathe and multiply.
One simple and effective practice is to always match the size of the container to the quantity of seed, so that the container is completely full of seed. Tap the container on the ground as it is filled, so the seeds are packed tightly together, and fill the container with as many seeds as possible. This will immediately limit the amount of air in the container.
The amount of oxygen can be further reduced in a number of ways. Air spaces between seeds can account for 40% to 60% (or even more) of the volume of the container, depending on the species. We can reduce this air by half by filling the spaces between the seeds with fine, dry sand, wood ash, or fresh Portland cement powder. The fine material is poured into the top of the almost-full bottle, which is tapped continuously until the bottle overflows and no empty spaces can be seen. This technique can also be used to fill empty space if there are not enough seeds to fill the bottle. If these materials are clean and dry (sand may need to be sterilized in an oven), our tests have shown that they do no harm to dry seed and they have been effective at controlling insects.
A slightly more difficult (but less messy) technique is to create a vacuum in the full bottle. Simple pumps can reduce the air pressure in a bottle to about half of ambient air pressure, thereby halving the amount of oxygen available.

The easiest way to make a vacuum pump is to get a bicycle pump, take the plunger out, flip over the leather cap on the end, cut the bottom end of the cylinder off and put the plunger back in again minus the spring (Figure 1C). This makes a very efficient vacuum pump for about $5. The mouth of the pump can then be applied directly to the bottle or the lid of larger containers filled with seed. However, in rural Cambodia, common bike pumps often have a more complex valve than the leather cap variety, and these are much more difficult to modify. Therefore, we developed a couple of other easy-to-make pumps.
The first is based on a 60 ml disposable syringe (Figure 1B). Like the bike pump, the end of the cylinder is sawn off cleanly and straight. In this case, the plunger doesn’t have its own exhaust valve, so we make one by drilling a small hole in the cylinder 1 cm above the bottom end, and secure a small square of rubber inner tube over the hole by wrapping electrician’s tape around the cylinder over the rubber, letting one edge of the rubber protrude from under the tape. A 60 ml disposable syringe costs about $0.50. If you would like to learn more about how to make a syringe vacuum pump, a video can be viewed on ECHO Asia’s YouTube channel.
The other pump is made from an 80 cm length of 35 mm 8.5 class PVC pipe(Figure 1A). This pipe matches nicely witha common leather pump cap that is sold in hardware stores. The cap is simply screwed onto the end of a straight length of bamboo with a roofing screw, soaked in engine oil, and then inserted into the pipe. An exhaust valve is created by again drilling some small holes near the bottom end of the pipe and a short section of bicycle inner tube is stretched over it. A flap of the inner tube can also be folded into the end, making a good soft end seal. The total cost of this pump is about $1.00.
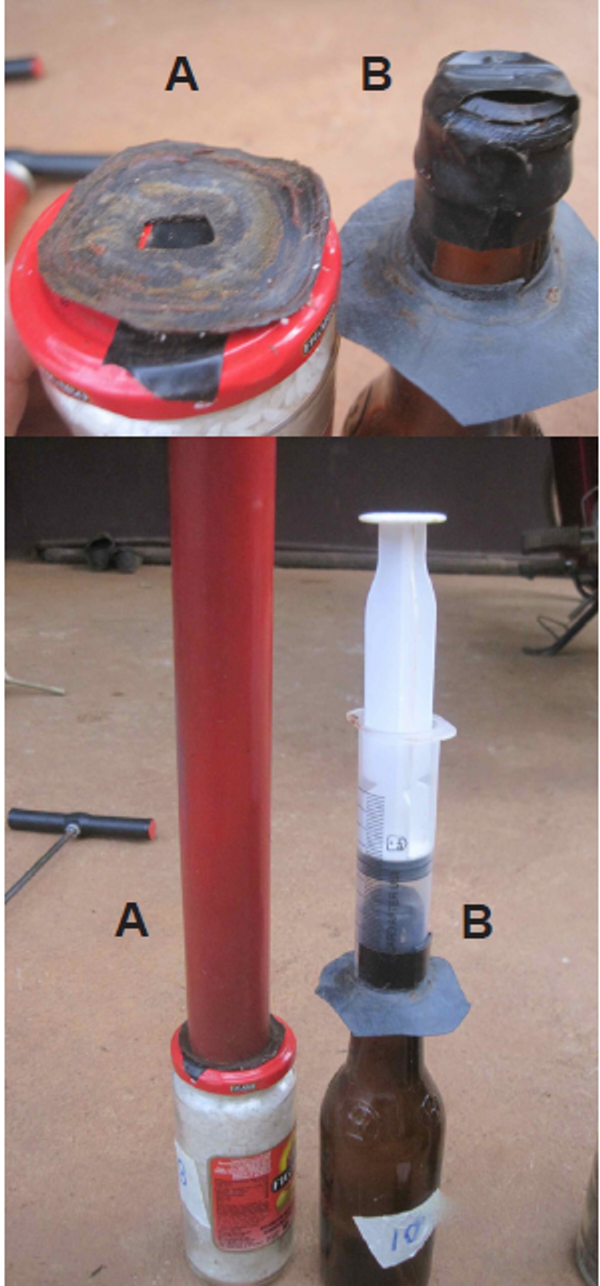
All three of these pumps are used in the same way. Make a one-way valve in the container’s lid by punching a small hole in the lid and covering it with electrician’s tape. Use an additional piece of inner tube rubber to form a seal with the pump(Figure 2). Jam jars and jerry cans have a top that is wider than the pump, so a piece of inner tube with a hole about 1 cm in diameter is laid over the top to line up with the one-way valve hole (Figure 2A). The end of the pump is pushed firmly against the rubber while pumping, to form a seal. For bottles with a narrow neck, the piece of rubber is pushed onto the neck at a position that allows the pump you are using to jam against the rubber on the neck during pumping, to form a seal (Figure 2B).
To reach maximum vacuum in small bottles, the big pumps only need to be drawn 2 or 3 times, while the syringe needs to be drawn 6 or more times. When maximum vacuum is reached, there will be significant resistance and the plunger will rapidly return to the bottom when released. Pumping to this point creates an air pressure in the bottle of about 20 in Hg (inches of mercury) below that of the outside air (Figure 3).

Figure 3: A pressure gauge checking the relative pressure of vacuum-sealed containers.
It is much easier to get a good seal between the pump and a bottle’s neck than it is with wide lidded jars or jerry cans. If the original bottle cap is lost or won’t seal back in place, a disc of thick inner tube can be cut to the same size as the top of the bottle, oiled on the underside, and then taped into position, leaving small spaces between the pieces of tape for air to escape (Figure 2B). Filling the bottle very full can help support the rubber disc, which, if too thin, will otherwise get sucked into the bottle.
CO2 Flushing
A third oxygen-reducing option we have explored is to actually replace all the air in the container with another gas. This method leaves almost no oxygen available and quickly kills insects. This technique has been used for large-scale fumigation of grain in storage, and some seed banks do this routinely-flushing all the seeds with pure nitrogen gas or other pure gases so that all insects die due to lack of oxygen.

Figure 4: Yeast generates CO2 in vessel C. This is captured in vessel B and displaces water into vessel A. For CO2 flushing, vessel C is replaced with a seed-filled storage vessel and water is added to A. This pushes water into vessel B to displace CO2 , injecting it into vessel C.
For resource-poor communities, carbon dioxide (CO2 ) or biogas can be easily generated at a low cost. In our experiments, we collected pure CO2 from a yeast-fermented sugar solution (Figure 4). The CO2 was gently injected into the bottom of the seed-filled vessel through a straw, and because CO2 is heavier than oxygen, it pushed the oxygen out the top of the bottle. A video about our CO2 flushing method can be viewed on ECHO Asia’s YouTube channel. Seeds stored for six months in this atmosphere at 24°C have shown no loss of viability compared to the pre-storage germination rate and compared to vacuum-packed controls (packed in laminated foil bags with a commercial vacuum packer). We have tried this method with several species so far with good results, but there is still some doubt as to whether it is safe for all species normally capable of long term storage (also known as orthodox seeds). Also, with this method, seed must be very dry before flushing with CO2 gas. Use caution and do your own experiments before sharing this technique with farmers.
We have also tried this flushing method using biogas (which is usually about 50% CO2 and 50% methane), and seeds stored this way are doing well. Flushing with biogas could potentially be used to fumigate large quantities of seed at very low cost, as manyfarms in Cambodia now have biogas generators digesting pig manure. This requires further research, as well, because some biogas sources may contain harmful levels of hydrogen sulfide (H2 S).
Current Trials
In addition to controlling insects, reduced oxygen may significantly extend the storage life of seeds by reducing the metabolic rates of the seeds and of microorganisms that might be present. We have a long-term trial comparing all the above simple treatments, plus some novel ones including zeolite desiccant beads and CALGLY, a liquid made of calcium chloride (CaCl2 ) and glycerol. These are all doing well, but we have only done germination tests at 3 months and 6 months so far. As we get results over the next few years, we will be able to give more definitive recommendations as to the best ways to store seeds for long periods without refrigeration.
Note that zeolite and CALGLY are not very suitable for resource-poor farmers, because they are more expensive and complicated to use than the other methods discussed in this article. However, they may be useful for organizations interested in seed banking in isolated areas. We will discuss our experience with zeolite and CALGLY in part 2 of this article.
Conclusion
The key to successful seed saving in the tropics with no refrigeration is to thoroughly dry seeds and then to keep them that way. Our experiments have shown that with a little effort and care, this can be achieved for all the orthodox species we have tested using a combination of shade and sun drying. Well-sealed glass or thick plastic bottles will continue to keep the seeds dry and will keep pests out. Applying any one of our three oxygen-reducing techniques will ensure that pests do not multiply in the stored seed. All of these techniques can be done inexpensively.
Further reading
In EDN 126, Abram Bicksler described similar experiments comparing a modified bicycle pump with other storage methods. The results are worth comparing. Also our modification to this pump makes it more efficient, simpler and more ergonomic. Chaper 7 of the FAO’s “A Guide to Forest Seed Handling” also gives excellent information on the intricacies of seed storage.