VITA 1600 Wilson Boulevard, Suite 500 Arlington, Virginia 22209 USA Tel: 703/276-1800 . Fax: 703/243-1865 Internet: pr-info@vita.org
Understanding Inorganic and Organic Fertilizers ISBN: 0-86619-241-7 [C]1985, Volunteers in Technical Assistance
PREFACE
This paper is one of a series published by Volunteers in Technical Assistance to provide an introduction to specific state-of-the-art technologies of interest to people in developing countries. The papers are intended to be used as guidelines to help people choose technologies that are suitable to their situations. They are not intended to provide construction or implementation details. People are urged to contact VITA or a similar organization for further information and technical assistance if they find that a particular technology seems to meet their needs.
The papers in the series were written, reviewed, and illustrated almost entirely by VITA Volunteer technical experts on a purely voluntary basis. Some 500 volunteers were involved in the production of the first 100 titles issued, contributing approximately 5,000 hours of their time. VITA staff included Maria Giannuzzi as editor, Suzanne Brooks handling typesetting and layout, and Margaret Crouch as project manager.
The author of this paper, VITA Volunteer Kenton K. Brubaker, is Professor of Biology and Director of International Agriculture at Eastern Mennonite College, Harrisonburg, Virginia. He received his doctorate in horticulture from Ohio State University and has had experience in tropical agriculture in Zaire, Bangladesh, and Haiti. His current research focuses on the use of organic fertilizers in vegetable production. The reviewers of this paper are also experts in agriculture. Roy Donahue has served as an agronomist and forester in Asia, Africa, and South America. J. Walter Fitts is President of Agro-Services International, Inc., an agricultural research, analysis, consultation, and planning firm in Orange City, Florida. Lee Fryer is President of Earth Foods Associates in Wheaton, Maryland.
VITA is a private, nonprofit organization that supports people working on technical problems in developing countries. VITA offers information and assistance aimed at helping individuals and groups to select and implement technologies appropriate to their situations. VITA maintains an international Inquiry Service, a specialized documentation center, and a computerized roster of volunteer technical consultants; manages long-term field projects; and publishes a variety of technical manuals and papers.
I. INTRODUCTION
Every farmer and gardener realizes that plants receive some of their substance from the soil. Just how much plants depend on soil fertility is not always obvious, however, because so many other factors also influence plant growth--water, sunlight, pests, and plant variety (genetics). In regions of the world where crop yields are extremely high, farmers add large amounts of fertilizer, usually in the form of a commercial product, which they purchase at considerable expense from a farm supply dealer. For example, in the corn belt of the central United States, yields of over 12 metric tons per hectare (200 bushels per acre) may be achieved by using hybrid corn, more than 125 kilograms (kg) of fertilizer per hectare (100 pounds per acre), and sometimes large amounts of irrigation water. Such a farmer may spend $500 per hectare for fertilizer to produce a crop worth $1,500 per hectare.
In much of the world such capital-intensive agriculture is impossible because of its high cost and often would be unwise due to the uncertainty of rainfall, insufficient length of growing season, or possible lack of demand for the crop at harvest. Nevertheless, addition of some fertilizer may be economically justified. The decision as to whether or not to use fertilizer will depend on the answers to the following questions:
- Will fertilizer substantially improve the yield or quality of the crop?
- Will the increased value of the crop cover the cost of the fertilizer?
- Are the risks associated with producing the fertilized crop (lack of rain, short growing seasons, pest damage, unstable market) low enough to justify the investment in fertilizers?
If the answers to all of the above seem to be "yes," then an additional set of questions should be asked:
- What type of fertilizer is needed, and how much?
- When and how should it be applied?
- Will the addition of fertilizer change plant growth in such a way that other problems may develop, like increased susceptibility to drought or pests, collapse of the plants due to stem weakness (called lodging in grain crops), or an undesirable change in quality such as taste, texture, or nutritional value?
Answers to these questions may not be easy to obtain since experience is often essential. Usually the farmer or gardener needs to experiment with fertilizer use in the field in order to learn the advantages or disadvantages. However, fertilizer experiments are often very difficult to interpret due to the many crop growth variables, so that information about experiments by local agricultural research stations may be highly desirable.
II. BASIC SOIL FERTILITY THEORY
LAW OF THE MINIMUM
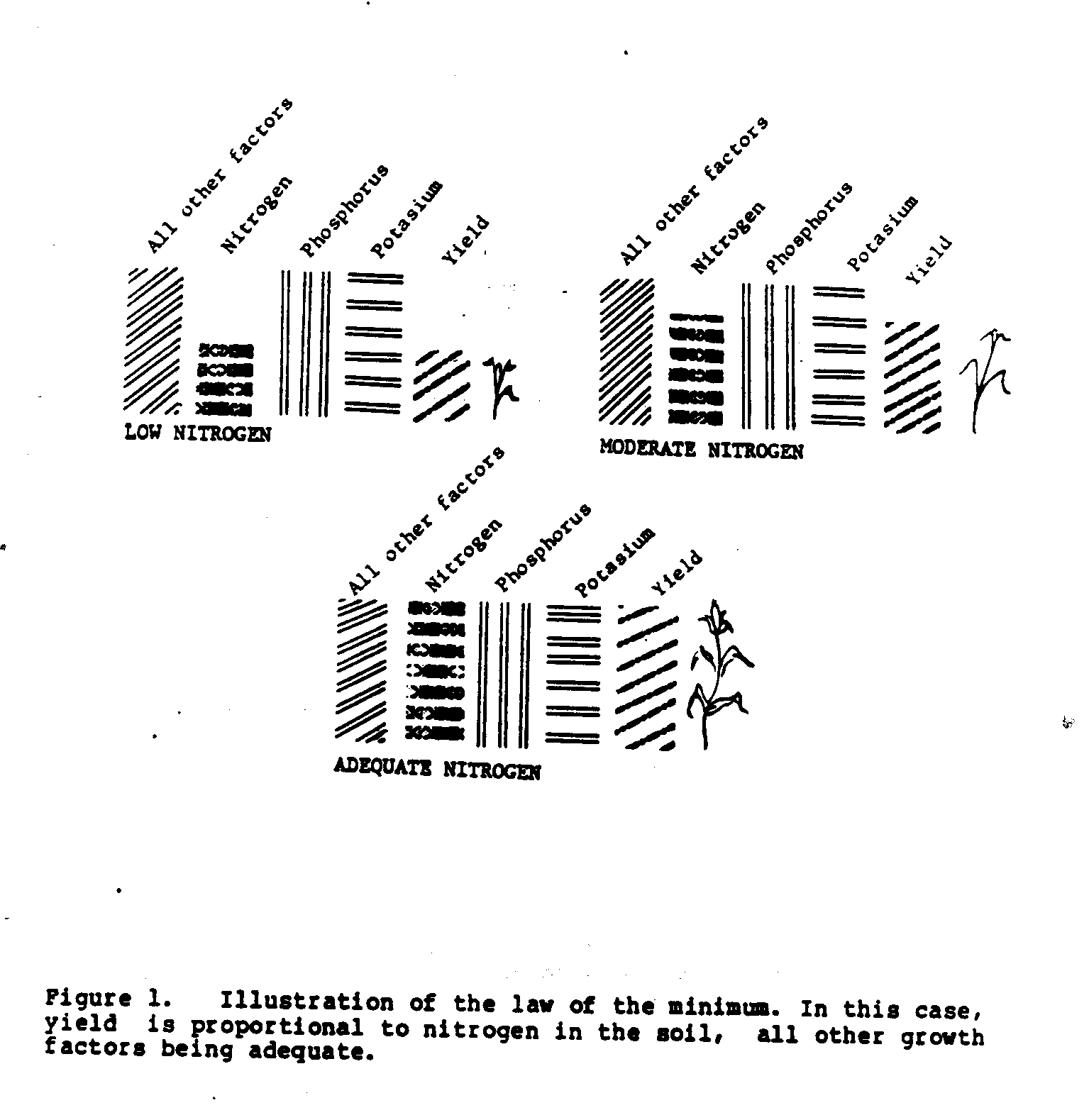
Crop growth and yield depend on a complex set of growth factors. The law of the minimum states that growth or yield is no higher than the factor that is most limiting to growth. Some factors, such as lack of water or obvious pest damage, are usually easy for the farmer to recognize. However, some limiting factors are not as easily detected, like the lack of an essential soil mineral element (e.g., nitrogen, phosphorus, or potassium), or the lack of good root growth due to poor soil drainage, or an insect or nematode eating the roots. Weed growth or soil erosion are other factors that may not be obvious to the grower and yet are most likely to limit yield.
The law of the minimum may also be applied to the restriction of growth due to the lack of just one soil mineral among the many that are essential. If we consider just three of the soil minerals--nitrogen, phosphorus, and potassium--and assume that all other growth factors are adequate, the one mineral that is not available in sufficient amount will be the one that limits the yield. Figure 1 illustrates the effect of three different soil nitrogen levels on yield.
FACTORS LIMITING CROP GROWTH
The first step in considering matters of soil fertility is to determine what factor or factors are most likely to limit crop growth and yield. For example, if lack of soil fertility is indicated, then one must find out which nutrient is lacking. Throughout the world, this element is most often nitrogen.
Several factors can limit plant growth:
- Lack of water
- Lack of sunshine - growing season too short - days too short - too cloudy, or crops shaded by trees
- Lack of oxygen for roots - soil water-logged, poor drainage - soil too compact, tight
- Soil too cold; may fail to warm up because of poor drainage
- Competition with weeds or other plants (too many plants)
- Pests and diseases that attack leaves, stems, fruits, or roots - insects (e.g., beetles, grasshoppers, aphids) - diseases (e.g., wilt, mosaic, blights, pythium) - nematodes - birds, rodents, and other animals
- Lack of soil nutrients due to - soil erosion with loss of most fertile layer - soil chemistry, especially improper soil pH(*) - leaching (removal of nutrients by the movement of water downward in the soil) or cropping removal)
- Crop variety, genetics
* pH indicates the acidity or alkalinity of the soil, and is based on a scale of about 4.0 to 6.5 (acid), 6.5 to 7.5 (neutral) and above 7.5 (alkaline), with the midpoint of 7 indicating the exact neutral soil condition. Most plants prefer a pH of about 6.5, which is slightly acid.
THE NATURAL CYCLE OF PLANT NUTRIENTS: THE NITROGEN CYCLE
Plant nutrients are neither created nor destroyed; they simply change their chemical form and move from place to place. The movement of nitrogen is interesting, complex, and usually the most crucial to plant growth, so we will deal with it in some detail in this paper.
The earth's atmosphere is the greatest reservoir of nitrogen; 78 percent of air is made up of this valuable element. Here it is present as a pure element, [N.sub.2], a form that most plants cannot use. The most important occurrence in plant nutrition is the process in which the elemental nitrogen of the air is converted into forms of nitrogen that most plants can absorb through their root systems. This process is called nitrogen fixation.
There are three ways nitrogen from the atmosphere can be obtained for use by plants (see Figure 2):
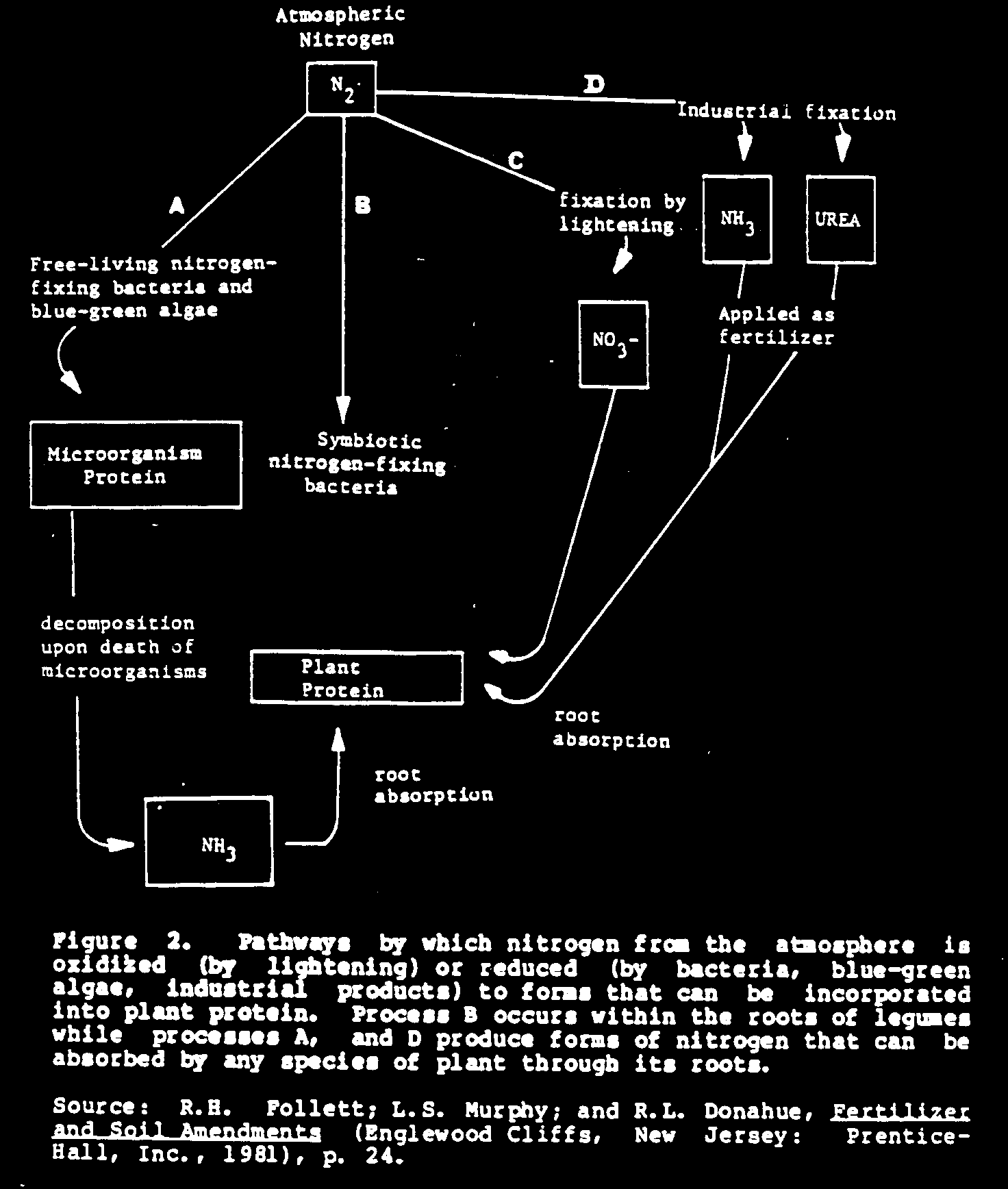
- capture of nitrogen by nitrogen-fixing bacteria or blue-green algae (a natural process);
- fixation of nitrogen by lightning in electrical storms (a natural process); and
- industrial fixation of nitrogen in fertilizer factories (an industrial process).
Nitrogen Fixation by Bacteria and Blue-green Algae
Certain bacteria and blue-green algae are naturally equipped to absorb inorganic, elemental nitrogen from the air and chemically change it through the addition of hydrogen (called chemical reduction) to the kind of nitrogen found in the organic molecules of plants and animals called protein. The nitrogen of protein is present as amine nitrogen, symbolized chemically as the amine group, -[NH.sub.2].
By maintaining a well-drained but moist soil, the free-living, nitrogen-fixing microorganisms can be cultivated, providing a cost.-free source of organic nitrogen. However, these bacteria must have an energy source on which to feed, such as straw or other plant residue, and this usually limits the amount of nitrogen they fix.
Other nitrogen-fixing bacteria live in specialized plant root tissues called nodules where they fix nitrogen and make it available to the host plant. Plants that contain nodules are usually legumes, which include members of the bean and pea family. A nodule that is active in fixing nitrogen will have a pink color if it is broken open and examined. The bacteria that live in nodules are called symbiotic because they benefit their host as well as get benefits from the host plant.
The water fern, Azolea, widely used in paddy rice culture, also has nitrogen-fixing microorganisms living in its tissues. These organisms make nitrogen available to both their natural host, the water fern, and to the rice plant. Thus, a farmer or gardener who grows legumes or other plants such as Azolea, which have nitrogen-fixing microorganisms associated with them, is able to convert free elemental nitrogen of the air into organic nitrogen of the crop plant.
Nitrogen Fixation by Lightning
Another natural process that converts elemental, atmospheric nitrogen into a form useful to plants is the electrical discharge, lightning, which occurs in thunderstorms. This process oxidizes nitrogen (combines nitrogen and oxygen) forming an inorganic nitrogen compound called nitrate ([NO.sub.3]-). This very water-soluble fertilizer is readily absorbed through the roots of plants. Electrical storms may contribute a substantial amount of nitrogen to the soil in some areas, although the heavy rainfall associated with such storms may tend to wash the nitrate out of the plant root zone fairly quickly. For this reason, a well developed root system, such as that of trees and grasses, is essential to capture this form of naturally-fixed nitrogen.
Industrial Nitrogen Fixation
A third process of fixing atmospheric nitrogen is accomplished by modern chemical technology in industrial facilities. This process uses natural gas and other hydrocarbon fuels to produce ammonia ([NH.sub.3]), ammonium ([NH.sub.4]+), and urea ([NH.sub.2] Q/[CNH.sub.2]), both useful forms of chemically reduced nitrogen. Ammonia can be considered inorganic nitrogen, while urea is an organic form of nitrogen because it contains carbon.
Table 1 summarizes the forms of nitrogen obtained from the earth's atmosphere.
SOME SOURCES OF NATURAL NITROGEN FERTILIZER
A rich and valuable natural source of nitrogen fertilizer is the oxidized, ancient deposits of bird and bat manure, known as guano, which occur in various locations around the world, especially in coastal regions and caves. The nitrogen in guano, which is collected and sold as fertilizer, is usually combined
Table 1. Forms of Nitrogen Obtained from the Atmosphere
Forms of Chemical Nitrogen Formula Comments
Atmospheric nitrogen [N.sub.2] Not available to plants except certain bacteria and blue-green algae.
Protein or amine -[NH.sub.2] Organic nitrogen produced by nitrogen nitrogen-fixing bacteria and blue-green algae and incorporated into the proteins of the microorganisms or the host plant when the microorganism is symbiotically associated with the host plant.
Nitrate nitrogen [NO.sub.3]- Inorganic nitrogen produced when lightning oxidizes atmospheric nitrogen.
Ammonium [NH.sub.4]+ Inorganic nitrogen produced by industrial fixation of atmospheric nitrogen.
Urea [NH.sub.2]-O/C-[NH.sub.2] Organic nitrogen produced by industrial fixation of nitrogen and hydrogen from natural gas, coal, or oil.
with potassium (K) or sodium (Na), forming potassium nitrate ([KNO.sub.3]) or sodium nitrate ([NaNO.sub.3]).
Another important natural source of nitrogen fertilizer is fresh or composted animal manure and human wastes. These are a complex mixture of several forms of nitrogen including urea (organic), protein (organic, mostly bodies of microorganisms), nitrates ([NO.sub.3]), ammonia ([NH.sub.3]), and, ammonium ([NH.sub.4]+) compounds. The value of animal and human manures as fertilizer depends on how the manure is handled, since it is a rich culture of bacteria, both living and dead, and various forms of nitrogen. If the manure is exposed to oxygen, the reduced forms of nitrogen (protein, ammonia, and urea) may be changed to nitrate by bacteria, or the population of bacteria may increase dramatically and incorporate most of the nitrogen as protein in their own cells. If the manure is handled so as to exclude oxygen (kept wet or tightly packed to exclude air), bacteria growth may be limited and the nitrogen will be mainly kept in the reduced forms (ammonia, ammonium, urea, and protein).
Whether or not the manure is kept under shelter to protect it from rain is also crucial since urea and nitrate nitrogen are easily washed out of the manure. Ammonia nitrogen is also readily lost to the air as it is quite volatile, but in the soil it changes to ammonium ([NH.sub.4]+) and is absorbed by clay.
Since the nitrogen content of animal manures is so easily lost, several management suggestions should be followed:
- Keep the manure under a roof to prevent leaching of nutrients that dissolve easily in water.
- Incorporate it into the garden or field as soon as possible to prevent loss of ammonia (or ammonium).
- Use a cement floor for storage to prevent loss of the liquid portion in which most of the urea and nitrate is found. Sufficient bedding to absorb the urine also saves urea.
- Compost human manures thoroughly to ensure that diseases and parasites are killed. (A description of appropriate methods of composting human wastes is beyond the scope of this paper.
Another source of nitrogen fertilizer is compost, a decomposing mixture of plant materials and manure. The nitrogen content of compost is usually very low unless it contains substantial amounts of legumes and manure and is handled with the same care as manure. The state of decomposition would also influence the percentage of available nitrogen it contains.
A final natural source of nitrogen fertilizer is the use of crops, especially legumes, as green manure. Crops that are naturally high in nitrogen are turned under and allowed to decay, thus releasing the nitrogen they obtained from the air through the activity of the symbiotic bacteria in their nodules.
Decomposition microorganisms play an important role in the natural cycle of nitrogen. Nitrogen may be lost from the plant-animal-soil phases of the cycle when certain soil microorganisms convert nitrates into elemental nitrogen, which then escapes back into the atmosphere. This loss seems to occur most readily when the soil is water-logged and microorganisms are forced to turn to nitrates ([NO.sub.3], [NO.sub.2], and NO) for their source of oxygen. Naturally, this loss of valuable fertilizer nutrients should be avoided if at all possible by seeing that the soil is well drained and thus well supplied with oxygen from the atmosphere. A well drained soil that permits good oxygen entrance can be produced by good cultural practices, especially by the addition of organic matter.
To sum up, then, management of the nitrogen cycle may be the most important activity a farmer carries out in relation to soil fertility. The lack of usable nitrogen is the most frequent cause of poor crop growth and yield in most soils around the world.
The nitrogen of the atmosphere is made available to plants only through nitrogen-fixation. The growth of both free-living and symbiotic bacteria can be managed to increase the amount of nitrogen in the plant growth cycle. Both symbiotic and free-living microorganisms grow well in moist, well-aerated soil.
The chemical state of nitrogen must be appreciated to manage the cycle successfully. Organic nitrogen in mainly protein, and the important waste product, urea. Such nitrogen is said to be chemically reduced or combined with hydrogen. Upon decomposition of protein and urea by bacteria, the nitrogen is released as a volatile gas, ammonia. This reduced form of nitrogen can be absorbed by plant roots, and it can also be converted by bacteria to an oxidized, non-volatile form, nitrate, which is also readily soluble and absorbed by plant roots.
Commercial fertilizers may be in the form of ammonia, ammonium salts, urea, or nitrate, all of which can be quickly utilized by plants. Urea quickly changes to ammonium and can then be absorbed by plants. Green manures and the protein components of animal manures must be changed to ammonium and nitrate before they can be absorbed by plants. Before conversion to soluble forms of inorganic nitrogen, the insoluble organic nitrogen of green and animal manures forms a reservoir Of nitrogen that will be released slowly (through bacterial decay) during crop growth. This slow release prevents its rapid loss during heavy rainfall. Highly soluble fertilizers like urea and nitrate are quickly lost when leaching occurs. Ammonia can also be lost as a gas, and nitrate can be changed to elemental nitrogen by oxygen-starved soil microorganisms and lost to the atmosphere.
INORGANIC AND ORGANIC FERTILIZERS
Inorganic fertilizers are generally salts of metals such as sodium, potassium, calcium, and magnesium. Ammonia can also act as a carrier of other inorganic nutrients when it occurs in the form of a salt of ammonia (ammonium salt). Several important inorganic fertilizer salts are listed in Table 2.
Table 2. Some Important Inorganic Fertilizer Salts
Name of Chemical Percentage Fertilizer Salt Formula of Nutrient (Elemental)
Ammonium nitrate [NH.sub.4] [NO.sub.3] 33.5% nitrogen
Di-ammonium [([NH.sub.4]).sub.2][HPO.sub.4] -21%, nitrogen phosphate 23% phosphorus Superphosphate Ca [(H.sub.2][PO.sub.4]).sub.2].[H.sub.2]O 20% phosphorus
Dolomite Mg[CO.sub.3] Ca[([CO.sub.3].sub.2] 10-20% magnesium
Source: N. Brady, The Nature and Properties of Soil (New York, New York: MacMillan and Sons Publishing Co., 1984).
Note that each of these fertilizer salts contains a certain percentage of the nutrient element based on the relative weights of all the atoms in the molecule.
Chemically speaking, organic molecules, and thus organic fertilizers, are those that contain carbon in organic form. The organic molecules we have considered so far are protein and urea. Living organisms contain many other important organic molecules including carbohydrates and nucleic acids. Any fertilizer whose nutrients are present mainly in organic molecules like urea, protein, or nucleic acids is called organic fertilizer. In general, such fertilizers (compost, manure, and cottonseed meal) have a low nutrient content and release these nutrients very slowly. This is because bacteria and fungi must first decompose the organic molecule for the nitrogen to be freed as ammonia or the phosphorus to be released as phosphate. Urea is an important exception to this general rule; it has a very high nitrogen content (46 percent) and is readily available for plant root absorption after a day or two when it has been converted by bacteria to ammonium salts.
Some examples of organic fertilizers with approximations of their nutrient content are given in Table 3.
The highly variable nutrient content of organic fertilizers makes their use more complicated than that of inorganic fertilizers, especially if the grower intends to achieve very high yields. This is because the content and form of nutrients is unknown, or only approximately known. Also, the generally low nutrient content of the organic fertilizer makes it necessary to add very large quantities of the fertilizer to the soil. The third complicating factor in the use of organic sources of nutrients is the slow release of most of the organic nitrogen and phosphorus. The organic matter must first be decomposed by soil microorganisms, which in turn must also die and decompose, before a substantial amount of these nutrients is available to plant roots. For example, suppose that the organic fertilizer to be used is compost, green manure, or animal manure--or a combination of any of these. If the approximate analysis of the organic material is 0.5-0.1-0.3 (nitrogen-phosphorus-potassium), how much would be needed per hectare to furnish the nutrients to produce 6 metric tons of corn (100' bushels per acre)?
One estimate suggests that the following amounts of available nutrients are needed to produce such a yield.
Nitrogen Phosphorus Potassium (Kilograms) (Kilograms) (Kilograms) Total needed to produce six metric tons of corn/hectare 168 67 134
Table 3. Total Nutrient Content Of Some Organic Fertilizers
Total Nutrient Content (Approximate Percentage)
Fertilizer Nitrogen Phosphorus Potassium
O Urea ([NH.sub.2] [CNH.sub.2]) 46 0 0 Guano (bat or bird fecal 10 2 2 deposits) Compost (highly variable) 0.1-0.3 <0.1 0.1-0.3 Green manure (legumes) 0.2-0.5 <0.1 0.2-0.4 Horse, cow, or hog manure 0.7 <0.l 0.5 Poultry manure 1.0 0.3 0.3 Sewage sludge 2-6 1-2 0.1-0.4 Dried fish scraps 6-10 2-4 -- Cottonseed meal 6-9 1-2 1-2 Bone meal 2-3 10-15 WOOD ASHES 0-1 2-6
Source: Florida Cooperative Extension Service, Organic Vegetable Gardening, Circular 375-A (Gainesville, Florida: University of Florida, Institute of Food and Agricultural Sciences, May 1973).
If we added 50 metric tons of organic fertilizer per hectare, the following amounts of nutrients would be supplied:
250 kg nitrogen, 50 kg phosphorus; and 150 kg potassium
However, only about 30-50 percent of the nitrogen and phosphorus would be available the first growing season due to the slow process of decomposition of the organic matter. About 50 percent or more of the potassium would be available. In conclusion, it becomes obvious that supplying all nutrients in organic form is a rather uncertain and labor-intensive practice. As a result, organic fertilizers may need to be supplemented with chemical fertilizers.
Application of 50 metric tons of organic matter to a hectare (500 kilograms/are(*)) is a huge job. Furthermore, availability of that much material may also be a problem, and working the organic matter into the soil may require a large expenditure of energy. Addition of large amounts of organic matter to the soil may also lead to a phenomenon known as "nitrate depression," where the soluble nitrogen gets incorporated in the bodies of soil decomposers until the carbon of the organic matter is decomposed. For this reason, the straw (cellulose) of organic matter should be decomposed rather thoroughly before it is used as fertilizer.
Adding nutrients to the soil in the form of organic matter is not easy, but it can be done. The process is an imitation of the natural fertility cycle of a forest, grassland, or pond. Experience and wise management plus a lot of hard work are essential to making the process work successfully.
Alternative methods of adding large amounts of organic matter should be evaluated. Composting is essential to decrease the carbon content of the plant material that is added to the compost heap, thus permitting more rapid release of the nitrogen and phosphorus when the material is added to the soil. Another important technique is to use the partially decomposed organic matter as a mulch, thus allowing the composting process to continue on the surface of the ground. The mulch that remains on the soil surface at the end of the growing season may then be incorporated into the soil as compost. A third alternative is to incorporate fresh or partially composted organic matter into the soil just before a fallow period, allowing soil microorganisms to begin decomposition during a winter or dry season period when crops are not growing. Little soil microorganism activity occurs during such a fallow period, but some beneficial decomposition does take place.
* One are = 100 square meters = .01 hectare.
COMMERCIAL FERTILIZER FORMULATION
Suppose we wanted to make a complete inorganic fertilizer, that is, one containing nitrogen, phosphorus, and potassium, all derived from inorganic fertilizer salts. If we mixed potassium nitrate and ammonium phosphate, we would have such a fertilizer.
To give a simple example, suppose we mixed 100 kilograms of potassium nitrate ([KNO.sub.3]) and 150 kilograms of ammonium phosphate [([NH.sub.4]).sub.2] [HPO.sub.4] to make 250 kilograms of complete fertilizer. Let us calculate how much of each element would be present in this batch of fertilizer.
Nitrogen Phosphorus Potassium (Kilograms) (Kilograms) (Kilograms)
100 kilograms KNO (14%N, 39%K) 14 0 39 150 kilograms (NH) HPO (21%N, 23%P) 31.5 34.5 0 250 kilograms 45.5 34.5 39
We can now calculate the percentage of each element (analysis) in this mixed fertilizer as:
Nitrogen = 45.5 kg/250 kg = 18 percent Phosphorus = 34.5 kg/250 kg = 14 percent Potassium = 39.0 kg/250 kg = 16 percent
We would label this an 18-14-16 fertilizer. In commercial trade, this would be considered a high-analysis fertilizer because it contains a fairly high content of nutrients and no filler.
Many commercial fertilizers, at least those that are relatively inexpensive, have a lower analysis, like 5-10-10. In such a fertilizer, the inert material (filler such as sand or sawdust) would be 75 percent of the weight. If one needed to transport the fertilizer a long distance, this non-nutrient weight should be considered. High-analysis fertilizers give more nutrients per kilogram but they often require special care in handling and storage. For example, they must be kept dry because the salts readily pick up water and so are packaged in plastic-lined bags and stored in dry areas. Anhydrous ammonia, a very high-analysis nitrogen fertilizer, is handled as a liquid under pressure in corrosion-resistant tanks. Many dry fertilizers are granulated and coated with clay and wax to make them easier to store and handle. The coating may also slow the release of the nutrients when added to the soil; this slower release may be desirable. Moreover, the inert material may contain some trace elements that may be absent in high-analysis fertilizers.
DETERMINING THE NEED FOR FERTILIZERS
Observation of Visual Symptoms
Under severe deficiency conditions, a trained plant nutritionist can diagnose the need for a particular fertilizer element by examining the growth of the affected plants and the plants' symptoms. For example, nitrogen-deficient plants are small and have a yellowish appearance, especially the lower leaves. Potassium-deficient plants may show dead tissue around the edges of lower leaves and other symptoms such as missing kernels in ears of corn. Iron-deficient plants usually show a marked yellow color (chlorosis) at the growing tips of the plant. However, the use of visual symptoms is not a reliable method of assessing the need for fertilizers. Many factors limiting plant growth (e.g., nematode damage or magnesium deficiency) will cause similar plant symptoms. Also, when several factors are involved, the visual symptoms can become very confusing. Even experts have difficulty identifying a deficiency by visual observations. Moreover, by the time visual symptoms occur, so much damage has already taken place that correction of the problem is too late to be of much value for the current crop.
Soil and Tissue Testing
Analyzing the soil before planting and testing appropriate tissues before visual symptoms occur are better methods of determining the need for fertilizers. Soil or tissue samples are usually sent to a central laboratory, which then gives advice on fertilizer needs. Portable kits are also available to test soil and tissues but require a good understanding of their use and limitations. In general, portable soil-testing kits are used best in conjunction with a standard soil and tissue testing laboratory.
Experimental Testing and Crop Yield
The best method of assessing the need for fertilizer is actual field trials in which various combinations of plant nutrients are applied to the soils and crops in question. Again, this procedure needs to be carried out with great attention to experimental design but finally becomes the basis for other techniques such as soil analysis. Such field trials are usually carried out by research centers. In most developing countries, a farmer or gardener can often determine the need for fertilizer by fertilizing only a part of a field or garden and observing the results.
III. ALTERNATIVE SYSTEMS OF CROP FERTILIZATION
NATURAL SYSTEMS USING SOIL-ENRICHING FALLOW
All successful crop production systems that do not rely on the addition of fertilizers must imitate the natural cycle that existed in the region before the land was cultivated and devoted to raising crops. This principle is most clearly seen in the "slash-and-burn" or "swidden" agricultural method of the tropics. With this practice, a forested area that appears to be suitable for cropping is first selected for clearing. The forest demonstrates its fertility by the vigor of plant growth, both trees and undergrowth. The farmer can possibly evaluate the yield potential by feeling, smelling, and tasting the soil, and by observing forest growth. A fertile soil feels soft and crumbly, smells somewhat like new-mown hay, and tastes slightly sour.
In the tropics, larger amounts of plant nutrients are stored in the existing vegetation than in the soil. With the "slash-and-burn" practice, this reservoir of plant nutrients is returned to the soil surface as ash through careful burning of the mass of vegetation. Burning may also help kill pests in the soil including weed seeds. A mixture of crops is then planted, including legumes as well as many other plants whose size and placement imitates the forest structure they have replaced.
After two or three years of crop production, the yield decreases to the point where weeding no longer seems practical and the field is allowed, or encouraged, to return to mature forest as rapidly as possible. Many slash-and-burn farmers cherish the sprouting trees that will regenerate the nutrient stores of the mature forest. The roots of these trees and vines will penetrate deeply into the soil and retrieve nitrogen and other soluble nutrients that will have leached from the topsoil during the brief period of cropping. This forest fallow (regrowth) may require 12-20 years to regenerate soil fertility. Certain practices such as the planting of tree legumes could possibly hasten this regeneration, but the cycle cannot be shortened too much or the soil will be permanently damaged. Unfortunately, population pressures in many areas force farmers to re-use fields before they have fully regenerated, and crop yields have declined accordingly.
Other cropping systems such as wet rice paddies also imitate the natural swamp ecosystem, but these may be associated with an annual flooding cycle, and so are not dependent on a vegetation regeneration process. The flooding brings a substantial quantity of nutrients from the eroding hillsides farther up the valley. Flooding also makes soil nutrients such as phosphorous more readily available.
CROP ROTATION WITH GREEN MANURES
A system widely practiced before about 1950 in the temperate agricultural regions is crop rotation. Here cash crops such as corn and wheat are rotated with soil building crops such as clover, alfalfa, or beans, usually soybeans. Some of the soil-improving crop may be removed as hay or, for beans, seeds to sell, but as much as possible is returned to the soil as a way of building up the nitrogen content of the field. Before the wide use of commercial fertilizers, this was one of the most important practices of temperate agriculture. In combination with the use of manure (the next alternative discussed), it is still practiced by a small group of farmers known as "organic" farmers. These farmers may also use limited amounts of commercial fertilizer (the last alternative described below).
COMBINING CROP PRODUCTION AND ANIMAL HUSBANDRY
Many farmers find that the incorporation of animals into their agricultural system is crucial to crop production. The manure from these animals is carefully placed on the fields. Gardeners, with a smaller area to cultivate, may incorporate animal manures into a composting system, thereby increasing the quantity and quality of the organic fertilizer they use to fertilize their gardens. Chinese farmers have developed especially intricate systems of using both animal and human manure (known as night soil) in the production of crops. The integration of hogs and fish into these systems is also crucial to food production programs.
To make compost, a partially decayed mixture of mostly plant
material, the following points should be kept in mind:
- Use plant residues as rich in nitrogen as possible and supplement with animal manure. Materials rich in nitrogen include legumes and animal materials (e.g., fish scraps).
- Chop as finely as practical and mix the materials from time to time, if you wish to achieve more rapid decomposition.
- Keep moist but not saturated so that air is available.
- Add superphosphate or rock phosphate to help prevent the loss of ammonia.
- Add a small amount of already partially decomposed compost or rich-garden soil to promote favorable decomposition. It will inoculate the compost with useful bacteria and fungi.
- Keep the compost heap large enough to ensure uniform heating but not so large that air is excluded (a minimum of about two square meters). A compost heap that is too small will not heat adequately enough to destroy weed seeds and pathogenic organisms.
APPLICATION OF COMMERCIAL FERTILIZER
When it is impossible or impractical to use natural methods of maintaining soil fertility, the addition of commercially produced fertilizers is necessary. They can also be used to supplement any of the above alternatives.
Applying the proper kind and amount of fertilizer is crucial, since these materials are highly concentrated and often expensive. The kind and amount of fertilizer must usually be determined experimentally and should be adapted to the soil and location. Usually the fertilizer is placed in the soil below and beside the seed so that the growing roots can quickly begin to feed on the nutrients. Under no circumstances should chemical fertilizers be mixed with seed; to do so will kill the germinating seed. Applications of fertilizers, especially nitrogen, may be spaced out over the growing season in regions of very high rainfall.
IV. CHOOSING THE BEST SYSTEM OF CROP FERTILIZATION
ADVANTAGES AND DISADVANTAGES OF THE FOUR ALTERNATIVE SYSTEMS
Natural-Soil Enriching Systems
On the plus side, these systems
- Are inexpensive because a free service of nature: forest growth, annual flooding, natural reseeding.
- Provide many benefits in addition to increasing soil fertility that the farmer may not even be aware of, such as recycling of trace minerals and pest control processes.
- Offer ecological stability and genetic diversity because they are part of a complex natural system with many plant species cooperating with one another.
On the other hand, such systems
- May require years to regenerate fertility, thus requiring a substantial percentage of land in fallow. Where a severe deficiency occurs, such as very low levels of phosphorous in the soil and soil-forminq materials, natural soil-enriching systems do not replenish these elements.
- Are difficult to manage if poor or undesirable tree or weed growth occurs.
- Are not easily adapted to mechanized crop production; thus, natural soil-enriching systems are labor intensive.
- Will not support large populations.
Crop Rotation with Green Manures
The advantages of crop rotation with green manures include:
- Free source of nitrogen through nitrogen-fixation, where legumes are grown in the rotation.
- Green manure crops control soil erosion and may control some weeds.
- Green manure crops not only improve soil fertility but also dramatically improve soil structure and increase organic matter content.
- May be combined with animal production.
Some of the disadvantages include the following:
- A considerable amount of land must be used for green manure, taking it out of production.
- Incorporating the green manure crop into the soil may require considerable animal or mechanical power to turn the soil.
- The cost of good seed may be prohibitive.
- Inoculation with suitable bacteria may be essential.
- Green manure crops often deplete soil moisture, leaving a dry soil for the succeeding crop.
Integration of Crop Production and Animal Husbandry
Integrated systems have a number of advantages. These include:
- Animals provide valuable manure; they can also graze on land unsuitable for cultivation and eat roughage unsuited for human consumption, turning these materials into manure and animal products. + Animals can help diversify the range of agricultural products and give work when crops do not require attention. For example, fences can be repaired and manure handled at times when work in the crop fields is not necessary.
- Draft animals help work the land and carry products to market. Cattle may also be driven to market for sale. + Animal products (meat, milk, cheese, eggs) improve the nutritional quality of the human diet.
- Animal manure will improve the composting process, furnishing nitrogen for microorganism growth and ensuring better completion of the decomposition process.
- Like green manures, animal manures also greatly improve soil structure.
On the other hand,
- Animals may be expensive and require special skills and resources not readily available, such as veterinary services and high protein feed supplements.
- Animals require that a certain amount of land be devoted to pasture or other animal feeds; this land must be fenced to protect crops.
- Animals require constant care, which may be difficult to provide during busy crop production periods.
- Animal manure may be a source of distributing weed seed, insects, and some disease organisms.
Application of Commercial Fertilizers
Some of the advantages of the use of commercial fertilizers are:
- A fertility program can be designed especially for a particular crop under specific soil conditions.
- By selecting the proper fertilizer, rapid or slow release of the nutrient can be regulated.
- High yielding plant varieties can be used, especially the so called "miracle hybrids." These new hybrid varieties are designed to produce higher yields in response to additional fertilizer and water. Their genetic potential has been increased through plant breeding techniques.
- Land that has been depleted of nutrients can be rapidly rejuvenated in many cases.
- Irrigated lands can be farmed intensively.
- Large urban populations can be sustained.
As with the other systems, commercial fertilizers have drawbacks. These include the following:
- The cash investment may be prohibitive.
- Often other supporting technologies are needed along with fertilizers, such as irrigation and pesticides, further increasing the cash investment. This means that a whole "package" of technology may be required as yields are increased through new programs of fertilization.
- The fertilizer may be applied incorrectly (excessive amounts, wrong type, incorrect placement, or wrong time).
- Commercial fertilizers add only nutrients; they do not improve the soil structure. Unless good soil structure is maintained, the soil will deteriorate, and increasing amounts of commercial fertilizers will be required to maintain a given level of production.
- Facilities for handling and proper storage of the fertilizer may be inadequate.
ASSESSMENT OF LOCAL CONDITIONS AND RESOURCES
In choosing a new crop fertilization system, or more likely, in modifying a current system, one must realistically assess local resources. First, it is important to analyze carefully the system currently being used. It may be useful to concentrate on the movement of nitrogen through the cycle, and note where improvements of nitrogen availability to plants can be achieved. Perhaps commercial nitrogen fertilizer could be applied on certain crops to find out if additional nitrogen will increase crop yield. It may also be useful to determine the value of a phosphorus or potassium fertilizer on each of the important crops in the system.
Second, the nature of the soil or soils in the region should be identified. Factors to consider here would be the depth, texture (soil particle size), structure (crumbs, blocks, plates), organic matter content, drainage, slope, and nutrient content of the soil, including the acidity or alkalinity (pH).
The third factor to consider is the suitability of the crop or crops to the local soils, rainfall, temperature, length of growing season, ease of production, and marketability. The proper arrangement of crops on the farm and the best planting and harvesting sequence also need to be assessed.
The final factor to be considered is the availability of sources of plant nutrients. Are local deposits of nutrient-rich materials available? If the pH needs to be modified, is ground limestone available locally? If organic matter is needed, are good sources available? How could animal husbandry be better utilized to furnish humus and nutrients to the soil?
If resources are not available locally, then nutrients may need to be imported into the region. The organization of such supply systems may be carried out by private businesses, the government, or community cooperatives. Again, careful assessment and management is necessary to make certain such resources are both appropriate and economically justified.
ADDITIONAL CONSIDERATIONS
Rainfall and Irrigation
Many of the new high-yielding crop varieties require large amounts of water and irrigation is often essential to increase yield. This may require great expense if water must be pumped from a well or river. Many agricultural development schemes have run into considerable difficulties as water supplies became depleted or fuel costs increased sharply. An additional consideration is the expense of leveling the land to allow efficient surface irrigation. Also, for some soils, farmers need to prevent the buildup of sodium and other salts caused by the evaporation of water after several years of surface irrigation.
Soil Texture and Drainage
Soil texture, which is the percentage of sand, silt, and clay particles in the soil, must be considered in the management of soil fertility. A sandy soil (coarse texture) will not hold fertilizer nutrients against leaching. Therefore, fertilizer should be added in small amounts and fairly frequently. However, such a loose soil is well drained and thus permits good aeration of both plant roots and soil organisms. Organic matter (humus) added to a sandy soil may increase the humus content and also the nutrient-holding capacity. Many tropical sandy soils will not hold humus for very long because of the extremely high rate of organic matter decomposition. For such soils, the amount of clay minerals is crucial since these tiny clay particles will hold most fertilizer nutrients by adsorption (physical and chemical attraction).
Silt particles, intermediate between sand and clay in size, are also intermediate in fertilizer-holding capacity. Soils with a high clay content may be tight and poorly drained, thus decreasing the oxygen availability to roots. The addition of organic matter to such soil will often greatly improve the crumb structure of the soil, permitting better water drainage and an increased supply of oxygen. Unless a soil is well-drained, addition of fertilizer will have little value in yield improvement.
Soil Reaction
Soil reaction refers to the hydrogen ion content of the soil, which can be measure using the pH scale. A pH of below 6.5 is considered an acid soil and is unsuitable for many crops. The addition of lime or limestone (calcium carbonate) will help replace the hydrogen ions on the soil particles with calcium, raising the pH to a desirable level. Again, the higher the clay content or organic matter in the soil, the more calcium is required to replace the hydrogen on the clay or humus particles. Some old soils that have been leached for centuries are highly acid and may require considerable treatment to make them suitable for certain crops. Such soils may be suited to what are called acid-loving crops (such as bermuda grass, cotton, cowpea, peanut, pineapple, sweet potato, coffee, and orchids).
Previous Experience and Available Plant Varieties
The importance of research experience cannot be overemphasized in considering the soil fertility system. Such experience is difficult to obtain because demonstrations and experiments in which just one variable at a time is being examined are hard to design, but there is no better way to determine plant fertility needs. When new varieties of plants are being considered for use in the cropping system, their response to soil fertility must be examined under each type of field condition. Such research should be done at an agricultural research center, if possible.
V. FUTURE DEVELOPMENT OF FERTILIZATION SYSTEMS
RESEARCH
New methods of supplying nutrients to plants are emerging. Particularly promising is the genetic modification of plants other than legumes to accept nitrogen-fixing bacteria into nodules on their roots. With the advent of this technology, a major milestone in plant nutrition will have been reached. Currently, however, this type of genetic engineering is proving to be more complex than first anticipated.
Continued research in genetic engineering may produce additional genetic potential in crop plant growth and yield. The revolutionary type of plant breeding using tissue culture and haploidy should make possible new genetic advances whose nature is still unknown. Tissue culture takes single cells from a plant and grows them into new plants. If these single cells come from tissue with one set of chromosomes (haploid), such as the cells that give rise to pollen grains, then the hidden or recessive genetic traits will appear. This helps plant breeders deal with one gene at a time.
Research on the interactions of plants in mixed culture (growing more than one crop in a field at a time) is still only in the beginning stages, mainly because the industrialized, monoculture type of cropping patterns have tended to overshadow the more labor-intensive mixed culture technology. Mixed culture requires more harvesting and hand weeding since machines cannot distinguish among the plants. As certain regions of the world concentrate more on multiple cropping (growing more than one crop together), the symbiotic effects of such systems will become better known. Symbiosis occurs when both crops benefit by being grown together. One crop may help the other (e.g., corn can support climbing beans), while in return the second crop may furnish nutrients to the first (beans fix nitrogen, which the corn may use).
ECONOMICS
The economics of food production in the future is a major puzzle for many persons attempting to forecast agricultural trends. The cost of industrially-based resources, so essential for much "modern" agriculture, is escalating rapidly. Many North American farmers find their labor-efficient products to be priced above the amount hungry nations can afford to pay. For this reason, the poorer countries are often advised to develop a national food policy of self-sufficiency, based on local soil fertility resources.
The population pressure in most nations of the world is a major threat to many agricultural systems, especially those requiring fallow and crop rotation (different crops in different seasons on the same field). In countries with land reform programs where landless peasants are becoming landowners, the problem of decreased production for export often follows. Economic pressures on the nation for increased export earnings often are felt by the new landowners in the form of federal decrees. For example, a national government may require farmers to grow export crops like coffee or bananas, rather than food crops for local use; often farmers will resent these decrees. Economic factors often frustrate such programs because the new farmers are unable to produce the export crop successfully. As a result, the land returns to the creditors and landlessness is again established.
There is a constant struggle for farmers to care for their land and their families while at the same time trying to adjust to international economic realities beyond their control. The maintenance and improvement of soil fertility is basic to farmers, economic survival. However, there is no guarantee of success because factors beyond individual control may render all efforts futile. In the last-analysis, the protection of soil fertility and the economic viability of the agricultural sector must be part of the food policy of every national government.
BIBLIOGRAPHY/SUGGESTED READING LIST
Brady, Nyle. The Nature and Properties of Soil. New York, New York: MacMillan and Sons Publishing Company, 1984.
Donahue, Roy L., Miller, Raymond W., and Shicklum, John C. Soils, An Introduction to Soils and plant Growth. 5th edition. Englewood Cliffs, New Jersey: Prentice-Hall, Inc., 1983.
The Fertilizer Institute. The Fertilizer Handbook. Washington, D.C.: The Fertilizer Institute, 1982.
Follett, Roy H., Murphy, Larry S., and Donahue, Roy L. Fertilizers and Soil Amendments. Englewood Cliffs, New Jersey: Prentice-Hall, Inc., 1981.
McCune, Donald L. Fertilizers for Tropical and Subtropical Agriculture. Muscle Shoals, Alabama: International Fertilizer (undated).
Olson, R.A. Fertilizer Technology and Use. Washington, D.C.: Soil Science Society of America, 1971.
United Nations. Fertilizers and Their Use. New York, New York: United Nations, 1978.