Introduction
Some tropical crops contain cyanogenic glycosides, toxic substances that release hydrocyanic acid (HCN; also referred to as cyanide or prussic acid) when cells are crushed. Consuming these plants without cooking them can cause cyanide poisoning, with varying effects depending on cyanide levels and how long a person or animal has been eating that plant. Cassava roots and leaves contain cyanogenic glycosides, so people whose diets are heavily dependent on cassava are especially at risk. Traditional methods to process and detoxify cassava roots include fermentation, prolonged soaking and boiling. Chaya leaves also contain cyanogenic glycosides; it is best to cook chaya leaves before eating them, to boil off the HCN rather than ingesting it. ECHO has previously written about cyanide in food plants (see the Further Reading section at the end of this article).
To determine if a plant is safe to consume, either by humans or livestock, a simple cyanide screening test is very helpful. At the 2014 ECHO International Agriculture Conference in Florida, Dr. Ray Smith provided ECHO with sample strips of Cyantesmo paper for screening plant material for HCN. A 2.5 cm (1 in.) strip of this paper is all that is needed to detect the presence of cyanide in a sample of plant material. Cyantesmo paper is available in a 5-m long roll for 49.50 US dollars from CTI Scientific (item 90604). One roll supplies enough 2.5-cm long paper strips for 200 tests. The paper does not have to be kept in a freezer, although Smith recommends that it be refrigerated.
Steps in carrying out a test
Dr. Smith supplied a set of instructions written by himself and Drs Cindy Gaskill and Michelle Arnold (all at the University of Kentucky). Those steps, outlined below, are reprinted with permission from Dr. Smith.
- Collect a large handful of leaves to be tested. (Note: if testing plant material to be used for animal forage, such as the leaves of Johnsongrass or sorghum-sudangrass, collect the whole plant that the animal will likely consume. Young shoots are the most toxic.)
- Tear leaves/forage into small pieces; also mash the plant material to cause additional plant cell injury. (You are simulating how plant material turns “mushy” when fresh leaves and stems are chewed.)
- Place the sample into a heavy-duty quart-sized zip-lock baggie (if these are not available, find a similar-sized container that seals well) containing a 2.5 cm (one-inch) strip of Cyantesmo paper taped to the inside of the bag toward the top (tape the paper at just one end of the strip; if tape covers the entire strip, you don’t get color change). Use gloves [e.g., disposable latex or nitrile gloves] in handling the paper. The bag should be approximately half full. Keep the plant material from directly contacting the paper strip, so that you can easily evaluate the strip for color change.
- Some plant juice should squeeze out when you mash the leaves. If the sample material is dry, you will need to add about 15 ml (1 tablespoon) of water to the baggie—enough water to dampen the material.
- Seal the baggie and place it in a warm area, such as on the hot hood of a vehicle directly in the sun. Often, just placing the baggie in direct sunlight will heat it enough to release cyanide gas, if the latter is present in the plant material. This field test should be performed outdoors in a well-ventilated area.
- Wait 10 minutes, and then evaluate the color of the test strip.
- If the strip turns dark blue, the sample is positive for cyanide. If the strip is the same very light green color as before adding the sample, the sample is negative for cyanide. Any blue color change indicates that some cyanide is present.
- This test is simply a screening test to determine whether or not cyanide can be generated from the sample being tested. The exact concentration of cyanide cannot be accurately measured using this method, but a sample that quickly turns the strip dark blue indicates that the plant could pose a significant risk for cyanide poisoning. As long as the sample is damp “to the touch” when placed in the baggie, a lack of color change in the test strip after 30 minutes means the sample poses minimal risk of cyanide poisoning.
Note: The shade of blue can darken over time, indicating that trace amounts of cyanide are being generated. Test strips should be evaluated after 30 minutes, if possible, to detect trace amounts of cyanide.
Multiple samples (3 to 4 is probably the most practical) should be tested to get a good representation of the field or source.
Disposal: The sealed baggie can be discarded in the garbage, or the baggie can first be opened and ventilated outdoors in a well-ventilated area. Do not breathe the fumes from the baggie, as cyanide gas could be released as soon as you open it. Emptied and rinsed clean baggies can be reused as long as they still seal well. The paper itself should not be handled without wearing disposable gloves.
A simple ECHO trial
Methods

Photo of leaves that were chopped (left) prior to boiling (right) (source: Tim Motis).
To gain some first-hand experience using Cyantesmo paper, I (Tim Motis) followed the steps above for cassava (‘Negrita’) and chaya (‘Estrella’) leaves. I probably collected more leaves per sample than necessary (filling a whole bag versus half a bagful). Also, I chopped the leaves (Fig. 1) but did not mash them, since I would not normally mash chaya leaves before boiling them for a meal. Each sample consisted of enough leaves to fill a quart-size ziplock bag, amounting to 85 g of fresh leaves. I inserted a piece of Cyantesmo paper into each bagful of leaves, waiting at least 10 minutes before I removed the test strip to take photos.
After testing raw chaya and cassava leaves, I collected, chopped, boiled and then tested additional samples—a fresh batch of leaves for each 5 minute increment of boiling time. At the end of each boiling period, I emptied the pan of leaves into a strainer placed underneath a faucet in a kitchen sink, passing cold water over the leaves to immediately remove the heat. I increased the boiling time until cyanide was no longer detected, for a total of 8 batches of cassava leaves and 5 batches of chaya leaves (Fig. 2).
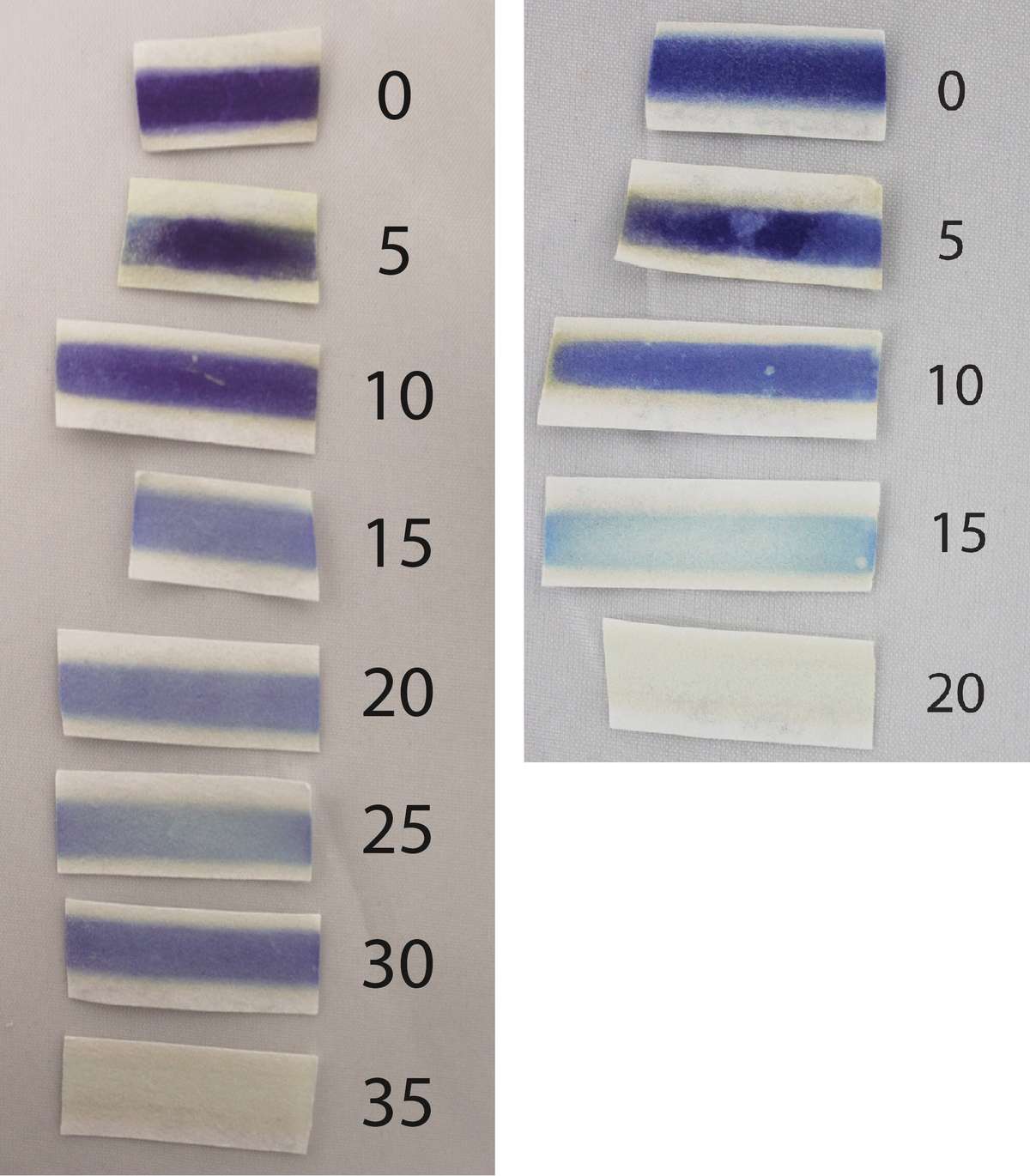
Figure 2: Color of Cyantesmo test paper after exposure to chopped leaves of cassava boiled from 0 (raw) to 35 minutes (left) and chopped leaves of chaya boiled from 0 (raw) to 20 minutes (right). The numbers in the photo below indicate the number of minutes that chopped, green leaves were boiled.
Results
With raw cassava and chaya leaves, the strips turned a dark blue color almost immediately, a strong indicator of the presence of cyanide (Fig. 2). For both crops, the shade of blue lightened considerably between 10 and 15 minutes of boiling time. However, it took 15 minutes longer for cassava than for chaya to reach the point where no blue color could be seen on a test strip.
Discussion
What does this mean? The results indicate that cassava leaves are safe to consume after 35 minutes of boiling time, and that chaya leaves can be eaten after 15-20 minutes of boiling. These results are hardly definitive, since only one sample was prepared for each boiling time. Still, these observations are comparable to other research findings. The 15-20 minute time frame for chaya is consistent with research showing that a boiling time of 15 minutes lowers HCN content to safe levels (Ross-Ibara and Molina-Cruz 2002). Also, many people boil chaya leaves for 15-20 minutes to reach a preferred level of tenderness. Where cassava leaves are eaten in West Africa, the young tender leaves are typically pounded and then boiled for up to 30 minutes (FAO 1999). The combination of pounding and boiling effectively reduces cyanide in the leaves to safe levels. In this experiment, the test strip after 30 minutes of boiling was surprisingly dark, which could have something to do with the ratio of older to younger leaves (assuming they differ in cyanide levels) in the sample.
Practical uses for this test
Cyantesmo paper could be used for a number of applications. The same series of boiling times could be tried for leaves from different varieties of cassava, which naturally tend to have differing levels of cyanogenic glycosides. Alternatively, the paper could be used to test how well cyanide is removed with other methods of food preparation, such as drying or frying. I have not tried it yet, but the paper should also work for assessing HCN released from mashed or cooked cassava roots. The test paper could also be used to determine the presence of cyanide in animal feed, by comparing HCN levels in different plant materials and resulting from different feed preparation methods.
Further Reading:
Food and Agriculture Organization (FAO). 1990. Oke, O.L. (edited by J. Redhead and M.A. Hussain). Roots, tubers, plantains and bananas in human nutrition. FAO (Rome). URL: http://www.fao.org/docrep/t0207e/t0207e08.htm
Food and Agriculture Organization (FAO). 1999. Bokanga, M (edited D. Mejia and B. Lewis). Cassava: Post-Harvest Operations. FAO (Rome). URL: http://www.fao.org/3/a-au998e.pdf
Ross-Ibarra, J. and A. Molina-Cruz. 2002. The Ethnobotany of Chaya (Cnidoscolus aconitifolius ssp. aconitifolius Breckon): A Nutritious Maya Vegetable. Economic Botany 56:350-365.
Articles in ECHO Development Notes:
Issue 81: Information on a cyanide testing kit developed by Dr. Howard Bradbury, using picrate paper and a color code to indicate cyanide levels in parts per million. Though we are unsure whether or not the kits are still available, an internet search with the terms “Bradbury cyanide method” will yield a number of related publications.
Issue 80: Information on cyanide levels in leaf protein concentrate made with chaya leaves.
Issue 89: Perspective on cyanogen content in cassava tubers/flour.
Cite as:
Motis, T. 2016. Cyantesmo Paper for Detecting Cyanide. ECHO Development Notes no. 130